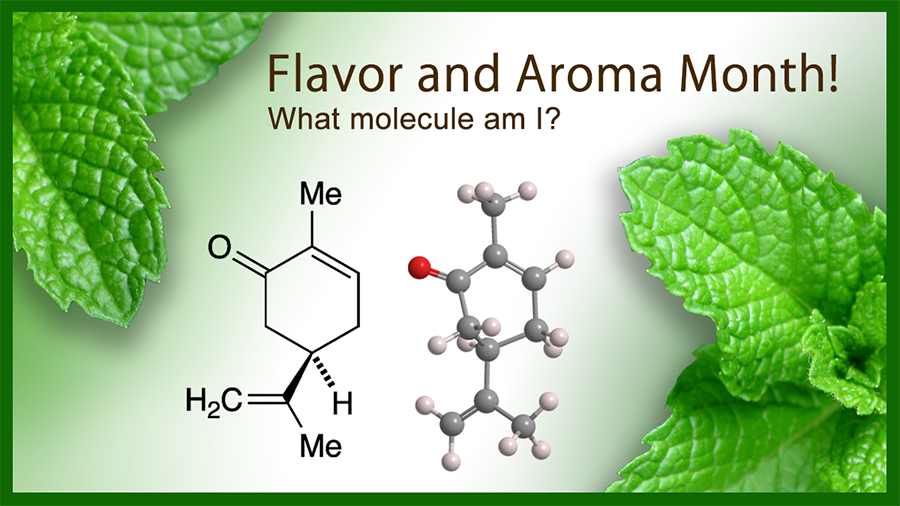
What molecule am I?
November is flavor and aroma month at MOTW!
Like limonene, the Molecule of the Week for November 1, 2021, carvone is a monoterpene that exists in nature as two enantiomers: (R)-carvone [aka (–)-carvone, L-carvone] and (S)-carvone [aka (+)-carvone, D-carvone]. The (R)-isomer is shown here.
Both enantiomers have well-recognized flavors and aromas. (R)-carvone has the flavor and aroma of mint; it is the dominant compound in spearmint oil. Like (R)-limonene, (S)-carvone1 is a key component of caraway and dill seed oils.
In 1841, Swiss chemist Eduard Schweizer isolated what eventually became known as (S)-carvone from oil extracted from caraway seeds (Carum carvi), from which carvone gets its name. Fifty years later, German chemist W. Kwaenick discovered its enantiomer in the oils of spearmint (Mentha spicata) and kuromoji (Lindera umbellata, a deciduous Asian shrub). In 1905, H. Waldbaum and O. Hüthig at what is now Technische Universität Dresden (Germany) reported that both isomers exist in gingergrass (Paspalum distichum) oil.
Both carvones are commercial products used in a variety of industries, primarily for their flavors and aromas. Natural (R)-carvone is used to flavor chewing gum and mint candies and to provide aromas in personal-care products, air fresheners, and aromatherapy oils. In 2009, the US Environmental Protection Acency registered it for use in insect repellents.
(S)-Carvone has fewer uses. Its presence in caraway oil adds to the flavor of kümmel, a liqueur popular in Europe. It is used in agriculture to prevent the premature sprouting of potatoes. Both isomers are used as starting materials for synthesizing complex terpenoids.
At 3800 t/year, the worldwide market for (R)-carvone is much larger than that of its (S)-isomer (10 t/year). Much (R)-carvone is extracted from natural mint, but about half of the commercial product is synthesized from (R)-limonene.
1. CAS Reg. No. 2244-16-8.
(R)-Carvone hazard information
| Hazard class* | GHS code and hazard statement | |
|---|---|---|
| Flammable liquids, category 4 | H227—Combustible liquid | |
| Acute toxicity, oral, category 4 | H302—Harmful if swallowed | |
| Skin sensitization, category 1 | H317—May cause an allergic skin reaction | |
| Short-term (acute) aquatic hazard, category 2 | H401—Toxic to aquatic life | |
*Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
(R)-Carvone fast facts
| CAS Reg. No. | 6485-40-1 |
| SciFinder nomenclature | 2-Cyclohexen-1-one, 2-methyl-5-(1-methylethenyl)-, (5R)- |
| Empirical formula | C10H14O |
| Molar mass | 150.22 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Boiling point | 228–230° C |
| Water solubility | 1.3 g/L |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

