What molecule am I?
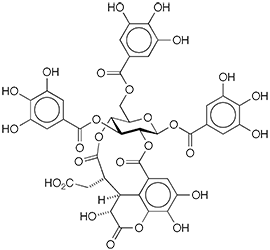
Chebulinic acid1 is a natural product in the family of ellagitannins, or hydrolyzable tannins, which consist of polyphenols surrounding a glucose center. The compound is found in plants such as Euphoria longana and Terminalia chebula (both tropical Asian fruit trees) and T. macroptera (a central African flowering tree).
Chebulinic acid has been known since 1911, when German scientist W. Richter described it under the name “eutannin”. In 2006, Hongxi Xu and co-workers at the Hong Kong Jockey Club Institute of Chinese Medicine reported the preparative isolation of it and the similar molecule chebulagic acid2.
Hydrolyzable tannins from the fruits and leaves of plants that contain chebulinic acid are used in traditional Chinese medicine. Consequently, the acid has been widely studied for its pharmacological properties.
In 2020, Sujit Basu and collaborators at Ohio State University (Columbus) and KPC Medical College (Kolkata, India) described how chebulinic acid can help reduce the pain of rheumatoid arthritis (RA). They noted that vascular endothelial growth factor-A (VEGF) induces angiogenesis in RA patients and that then-current anti-VEGF agents cause hypertension and other cardiovascular complications. They investigated reports that chebulinic acid can inhibit VEGF activity and found that it is a safe, potent antiangiogenic agent for treating RA.
Two years later, Bhawna Chopra and colleagues at Guru Gobind Singh College of Pharmacy (Haryana, India) reviewed the pharmacological properties of chebulinic acid and related ellagitannins. They examined biological properties, including antitumor activity and antiatherogenic, antifibrotic, anti-inflammatory, antiulcer, antioxidant, hepatoprotective, antidiabetic, and antiviral effects attributable to ellagitannins and recommended further studies in these pathology areas.
Also in 2022, Suman Tapryal and fellow investigators at the Central University of Rajasthan (Ajmer) and the National Institute of Virology (Pune, both in India) reported other benefits of chebulinic acid. Noting that the acid had showed the ability to inhibit herpes simplex virus-2 infections, they used in vitro and in silico methods to determine whether it is active against the dengue and chikungunya viruses. Their results indicated that chebulinic acid was ineffective against chikungunya but showed strong inhibition of dengue in the early stage of its infection cycle.
1. SciFinder: 7,11-methanopyrano[4,3,2-kl][2,5,8]benzotrioxacyclotridecin-4-acetic acid, 2,3,3a,4,5,7,8,10,11,13-decahydro-3,15,16-trihydroxy-2,5,13-trioxo-10,17-bis[(3,4,5-trihydroxybenzoyl)oxy]-8-[[(3,4,5-trihydroxybenzoyl)oxy]methyl]-, (3S,3aS,4S,7R,8R,10S,11R,17S)-.
2. CAS Reg. No. 23094-71-5.
Chebulinic acid hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 4 | H302—Harmful if swallowed | |
| Acute toxicity, dermal, category 4 | H312—Harmful in contact with skin | |
Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
Acute toxicity, inhalation, category 4 | H332—Harmful if inhaled | |
| Long-term (chronic) aquatic hazard, category 1 | H410—Very toxic to aquatic life with long lasting effects | |
*Compilation of multiple safety data sheets. Some SDS report “not a hazardous substance or mixture”.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
Molecule of the Future
Sorangiolide A1 is one of two metabolites2 isolated in 1995 by Gerhard Höfle and co-workers at the German Research Center for Biotechnology3 (Braunschweig) from the bacterium Sorangium cellulosum4. The authors reported that the sorangiolides showed some antibiotic activity against Gram-positive bacteria, including Staphylococcus aureus.
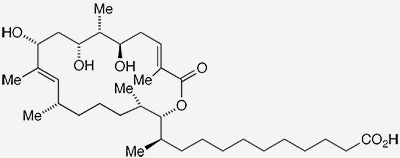
In October 2023, Rajib Kumar Goswami and colleagues at the Indian Association of Cultivation Science (Kolkata) reported a total synthesis of sorangiolide A. The researchers used several key techniques in their synthetic sequence, including a Julia–Kocienski olefination. They plan to use a similar process to make sorangiolide B, as well as structurally simpler analogues of the sorangiolides, to be used in medical research.
1. CAS Reg. No. 164177-51-9.
2. The other is sorangiolide B, CAS Reg. No. 164177-52-0.
3. Now named the Helmholtz Center for Infection Research.
4. S. cellulosum is a type of myxobacterium, a group of “slime” or “gliding” bacteria.
Molecule of the Future
Once a month we bring you a newly discovered or developed molecule that has important implications for the future of chemistry or society in general. Look for it the third week of each month. Learn more about this month's Molecule of the Future below.
We're looking for more molecules of the future!
Do you have a suggestion for the next molecule of the future? Send your idea to MOTW.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Chebulinic acid fast facts
| CAS Reg. No. | 18942-26-2 |
| Empirical formula | C41H32O27 |
| Molar mass | 956.68 g/mol |
| Appearance | White powder |
| Melting point | 234 °C |
| Water solubility | Slight |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

