What molecule am I?
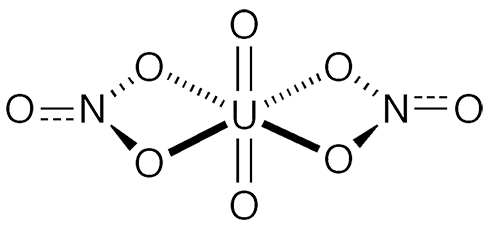
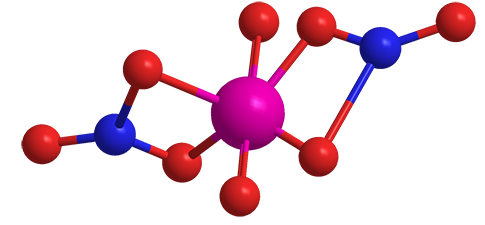
Uranyl nitrate is an inorganic salt with the formula UO2(NO3)2. It is highly soluble in water and is often marketed as its hexahydrate1 or in solution. As the hazard information table shows, it is an extremely hazardous substance.
Uranyl nitrate appeared early in the chemical literature in an 1890 article by Czech chemist Jaroslav Formánek, who wrote about uranyl chromate2 and some of its double salts. Five years later, Fanny R. M. Hitchcock at the University of Pennsylvania (Philadelphia) extensively described tungstates and molybdates of rare earths, including uranyl salts.3
Several applications of uranyl nitrate include precursor to uranium glazes in pottery and tiles, reagent in analytical chemistry, stain for microscopy, and photography intensifier (now obsolete). But its primary use is in nuclear fuel reprocessing, in which spent uranium fuel is dissolved in nitric acid to form uranyl nitrate, which is extracted into tributyl phosphate4. It is then converted to uranium hexafluoride5 and eventually to enriched uranium.
1. CAS Reg. No. 13520-83-7.
2. CAS Reg. No. 794448-03-6.
3. The article was based on Hitchcock’s PhD thesis.
4. CAS Reg. No. 126-73-8.
5. CAS Reg. No. 7783-81-5
Uranyl nitrate hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Oxidizing solids, category 2 | H272—May intensify fire; oxidizer | |
| Acute toxicity, oral, category 1 | H300—Fatal if swallowed | |
Acute toxicity, dermal, category 5 | H313—May be harmful in contact with skin | |
Skin corrosion/irritation, category 3 | H316—Causes mild skin irritation | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Acute toxicity, inhalation, category 1 | H330—Fatal if inhaled | |
| Germ cell mutagenicity, category 2 | H341—Suspected of causing genetic defects | |
| Carcinogenicity, category 1A | H350—May cause cancer | |
| Specific target organ toxicity, single exposure, category 1 | H370—Causes damage to organs | |
| Specific target organ toxicity, repeated exposure, category 1 | H372—Causes damage to organs through prolonged or repeated exposure | |
| Long-term (chronic) aquatic hazard, category, category 2 | H411—Toxic to aquatic life with long lasting effects. | |
*Compilation of multiple safety data sheets.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
MOTW updates
Coumarin1 was the Molecule of the Week for December 14, 2009. It is a natural product found in plants such as lavender oil and sweet clover. Its uses range from perfumes to rodenticides.
Earlier this month, Margaret Sunde, Elizabeth J. New, Amandeep Kaur, and collaborators at the University of Sydney and Monash University (Melbourne, both in Australia) reported a use for coumarin in research related to Alzheimer’s and Parkinson’s diseases. They developed a fluorescent coumarin-based two-sensor array that can correctly discriminate among four different amyloids implicated in amyloid-related pathologies with 100% classification. They also applied the array to mouse models of Alzheimer’s disease and found that it could discriminate between samples from mice corresponding to early (6-month) and advanced (12-month) stages of Alzheimer’s disease.
4-Vinylanisole2 (4VA. aka 4-methoxystyrene) was the Molecule of the Week for November 2, 2020. It is an aroma compound particularly known as a volatile component of a French brandy. In 2020, Chinese researchers reported that 4VA is a locust pheromone that causes the insects to swarm.
This month, Hong Pan, Daoyi Guo, and co-workers at Gannan Normal University (Ganzhou, China) described a de novo biosynthesis of 4VA. They used engineered Escherichia coli as the host organism to synthesize the pheromone from erythrose-4-phosphate3 and phosphoenolpyruvate4. The authors state that their synthetic method is an improvement over current chemical methods for producing 4VA and would increase the supply of the compound for capturing locusts and monitoring their population dynamics.
1. CAS Reg. No. 91-64-5.
2. CAS Reg. No. 637-69-4.
3. CAS Reg. No. 138-08-9.
4. CAS Reg. No. 585-18-2.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Uranyl nitrate fast facts
| CAS Reg. No. | 10102-06-4 |
| CA Index Name | Uranium, bis(nitrato-κO)dioxo- |
| Empirical formula | N2O8U |
| Molar mass | 394.04 g/mol |
| Appearance | Yellow-green, hygroscopic crystals |
| Melting point | 60 °C |
| Water solubility | 1220 g/L (20 °C) |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

