What molecule am I?
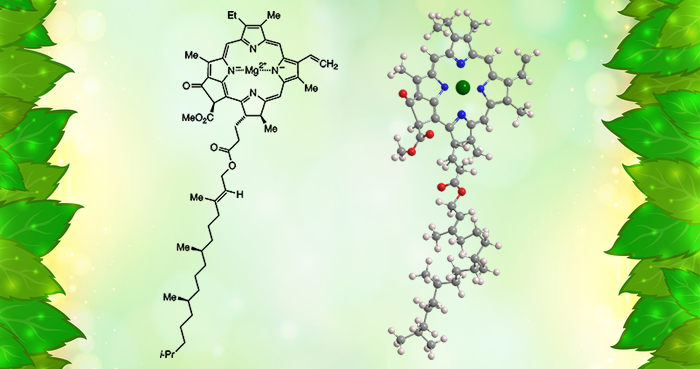
Chlorophyll is the green pigment in plants, algae, and cyanobacteria that is essential for photosynthesis. Its central structure is an aromatic porphyrin or chlorin (reduced porphyrin) ring system with a sequestered magnesium atom. A fifth ring is fused to the porphyrin.
Chlorophyll is not a single molecule: There are at least six varieties that have various side groups on the rings. In most chlorophylls, one of the groups is a long phytyl ester chain.
Chlorophyll a, shown here, is called the “universal” chlorophyll because it is present in almost all photosynthetic organisms. It has been known since 1817, but scientists did not realize that it contains magnesium until 1906.
By far the most important “application” of chlorophyll is photosynthesis; but it has also been used as a green coloring agent in foods, cosmetics, soaps, and alcoholic beverages. Its ester side chain can be cleaved to obtain phytol, an alcohol used in the synthesis of vitamins E and K1. It’s even been tried as an antiknock additive for gasoline.
Why is chlorophyll green? The bonding in many metal–organic coordination compounds causes them to absorb some wavelengths of white light while reflecting others. In the case of chlorophyll, light wavelengths in the blue and red regions of the spectrum are required for the pigment to do its business. Chlorophyll absorbs them; but it does not need to use green light, which is reflected to produce the intense green color of leaves.
January 26 is National Green Juice Day. (Yes, there is such a thing.) It’s a perfect time to get your fill of chlorophyll.
Chlorophyll hazard information
| GHS classification*: not a hazardous substance or mixture |
*Globally Harmonized System of Classification and Labeling of Chemicals.
Chlorophyll fast facts
| CAS Reg. No. | 479-61-8 |
| Empirical formula | C55H72MgN4O5 |
| Molar mass | 893.51 g/mol |
| Appearance | Green solid |
| Melting point | See “MOTW challenge” |
| Water solubility | Insoluble |
MOTW Challenge
Literature reports of chlorophyll a’s melting point vary widely: from 66–67 ºC to 117–120 ºC to ≈152 ºC (with decomposition). The last one appears to be the most widely accepted. But why the diverse findings? If you know, send the answer to motw@acs.org.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.


