What molecule am I?
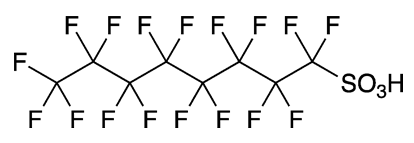
Perfluorooctanesulfonic acid, usually abbreviated to PFOS, was once a widely used surfactant in fabric protectors, firefighting foams, and photolithographic chemical mixtures. It was introduced in 1949 by 3M Co. (then known as Minnesota Mining and Manufacturing Co.).
By 1968, traces of PFOS began to appear in human blood. 3M began to phase it out in 2000, but it and similar perfluorinated compounds continue to be produced in China.
Because of the multiple hazards imposed by PFOS and its cousins (see hazard information box), efforts have been made to develop lower fluorine content or fluorine-free foams for several years. Specifically, perfluorinated C6 surfactants have lower toxicological and environmental profiles than the C8s. Some of the C6s meet US military foam standards.
Thus far, the C6 products have significantly outperformed fluorine-free surfactants. But the pressure is on to develop better non-fluorine foams because last year Congress passed, and the president signed, an act that allows civilian airports to use these foams to fight fires.
Perfluorooctanesulfonic acid hazard information
| GHS classification*: corrosive to metals, category 1 | |
| H290—May be corrosive to metals | |
| GHS classification: acute toxicity, oral, category 3 | |
| H301—Toxic if swallowed | |
| GHS classification: skin corrosion, category 1B | |
| H314—Causes severe skin burns and eye damage | |
| GHS classification: serious eye damage, category 1 | |
| H318—Causes serious eye damage | |
| GHS classification: acute toxicity, inhalation, category 4 | |
| H332—Harmful if inhaled | |
| GHS classification: carcinogenicity, category 2 | |
| H351—Suspected of causing cancer | |
| GHS classification: reproductive toxicity, category 1A | |
| H360—May damage fertility or the unborn child | |
| GHS classification: hazardous to the aquatic environment, acute hazard, category 2 | |
| H401—Toxic to aquatic life | |
| GHS classification: hazardous to the aquatic environment, long-term hazard, category 2 | |
| H411—Toxic to aquatic life with long-lasting effects | |
*Globally Harmonized System of Classification and Labeling of Chemicals. Explanation of pictograms.
Perfluorooctanesulfonic acid fast facts
| CAS Reg. No. | 1763-23-1 |
| Empirical formula | C8HF17O3S |
| Molar mass | 500.13 g/mol |
| Appearance | White powder |
| Melting point | Not available |
| Boiling point | 258–260 ºC |
| Water solubility | 680 mg/L |
MOTW update:
April 17, 2023
Cannabidiol1 (CBD) was the Molecule of the Week for February 6, 2017. It is a non-psychoactive marijuana constituent that is useful for controlling pain and other medical conditions. Perfluorooctanesulfonic acid2 (PFOS) was once a widely used surfactant; but because it became an environmental pollutant—even appearing in human blood—it was removed from the market.
What’s the connection between CBD and PFOS? Earlier this month, Hang Yin, Shu Li, and co-workers at Northeast Agricultural University (Harbin, China) reported that administering CBD can attenuate PFOS-induced heart injury. In a mouse study, the researchers found that CBD alleviates myocardial cell apoptosis caused by PFOS by restoring antioxidant capacity, mitochondrial function, and energy metabolic homeostasis to the cells.
1. CAS Reg. No. 13956-29-1.
2. CAS Reg. No. 1763-23-1.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

