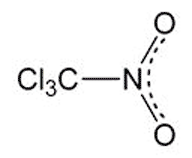
Chloropicrin, or trichloronitromethane, is a dense, pale yellow liquid that decomposes when heated to ≥112 ºC. Its property as a lachrymator prompted Germany to use it as a tear gas against the Allied forces during World War I—one of the first uses of a chemical weapon.
In 1848, Scottish chemist J. Stenhouse prepared chloropicrin by treating picric acid with sodium hypochlorite. Nitro groups were the only parts of the picric acid molecule to be used in chloropicrin, but the “picrin” name stuck. Today, chloropicrin is manufactured by the reaction between nitromethane and sodium hypochlorite.
Chloropicrin has long been used as an agricultural soil fumigant because it is effective against a broad spectrum of fungi, nematodes, and insects. In 2008, the US Environmental Protection Agency reapproved it for this use, but restricted its scope and required strict protections for farm workers. Additional restrictions have been applied since then. In early 2015, the California Department of Pesticide Regulation added even more stringent regulations on chloropicrin to protect workers and residents who live near fields where it is used

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

