What molecule am I?
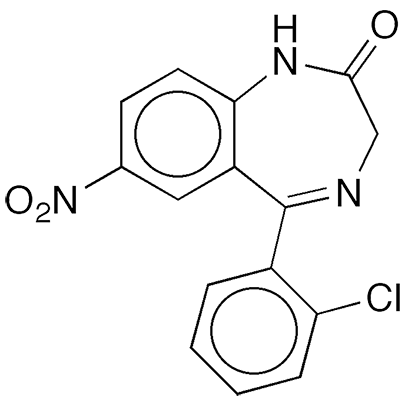
Clonazepam is a benzodiazepine-class medication whose synthesis was reported in 1962 by Leo H. Sternbach and co-workers at Hoffmann–LaRoche (Nutley, NJ).1 The report was the tenth in a series on quinazolines and 1,4-benzodiazepines. Sternbach was a pioneer in the development of the psychotherapeutic benzodiazepines, the best known of which is former Molecule of the Week diazepam2 (Valium).
Clonazepam was originally developed as an anticonvulsant; but it is more frequently used to treat anxiety, seizures (including those associated with epilepsy), panic disorder, and some psychoses. Although the drug is considered to be nonhazardous, the US Drug Enforcement Administration classifies it as a Schedule IV controlled substance because abusing it can lead to psychological dependence.
Sternbach’s clonazepam synthesis ended with nitration of the previously unsubstituted benzene ring. The current manufacturing method, according to R. S. Vardanyan and V. J. Hruby in Synthesis of Essential Drugs, is similar to Sternbach’s synthesis: five steps starting from 2-chloro-2′-nitrobenzophenone and ending with the same nitration step.
Molecules from the journals
Mycosporine-like amino acids (MAAs) are UV-absorbing materials found in organisms such as cyanobacteria, fungi, and algae1 that are exposed to large amounts of UV radiation. One MAA, shinorine2, was isolated in 1980 by Isami Tsujino, Kazuo Yabe, and Isao Sekikawa at Hokkaido University (Hakodate, Japan) from the red alga Chondrus yendoi. Shinorine is an active ingredient in some cosmetics and skin-care products, but it is available only in low yields from the alga Porphyra umbilicalis.
This past December, Soo-Jung Kim and colleagues at Chonnam National University (Gwangju), Kyungpook National University (Daegu), and Bio-FD&C Co. (Incheon, all in the Republic of Korea) developed a sustainable method for producing shinorine from lignocellulosic biomass by metabolically engineering the yeast Saccharomyces cerevisiae.
Prorocentin3 is a complex polyketide–polyolefin isolated in 2005 from the marine dinoflagellate Prorocentrum lima by Chung-Kuang Lu and collaborators at the National Research Institute of Chinese Medicine (Taipei) and other Taiwanese institutions. The authors elucidated a structure for prorocentin; but some researchers have since insinuated that the structure might need revision.
This January, Alois Fürstner and co-workers at the Max Planck Institute for Coal Research (Mülheim an der Ruhr, Germany) reported total syntheses of Lu et al.’s structure and what they believe is the most plausible amended structure. The difference between the two structures is the position of a hydroxyl group.
1. Speaking of algae, the theme of this April’s Chemists Celebrate Earth Week is “The Curious Chemistry of Amazing Algae”.
2. CAS Reg. No. 73112-73-9.
3. CAS Reg. No. 865536-64-7.
Molecules from the Journals
MOTW briefly describes noteworthy molecules that appeared in recent ACS journal articles. See this week's
edition below.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Clonazepam
fast facts
| CAS Reg. No. | 1622-61-3 |
| SciFinder nomenclature | 2H-1,4-Benzodiazepin-2-one, 5-(2-chlorophenyl)-1,3-dihydro-7-nitro- |
| Empirical formula | C15H10ClN3O3 |
| Molar mass | 315.71 g/mol |
| Appearance | White crystals or powder |
| Melting point | 238–240 °C |
| Water solubility | <0.1 g/L (25 °C) |
Clonazepam hazard information
| Hazard class* | GHS code and hazard statement |
|---|---|
| Not a hazardous substance or mixture |
*Globally Harmonized System (GHS) of Classification and Labeling of Chemicals.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

