What molecule am I?

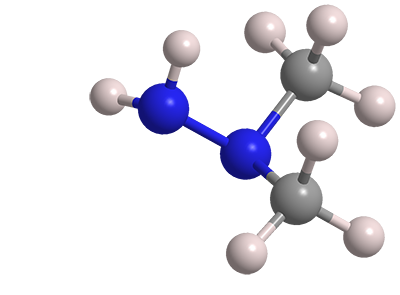
1,1-Dimethylhydrazine, frequently called unsymmetrical dimethylhydrazine (UDMH), is the third member of the hydrazine family to appear in Molecule of the Week. The first two were the parent molecule hydrazine in 2010 and methylhydrazine earlier this year. All these highly toxic hydrazines are used in rocket fuels.
Australian chemist Henry H. Hatt reported a synthesis of the hydrochloride salt1 of UDMH in the 1936 edition of Organic Syntheses. Hatt treated dimethylamine with nitric acid to produce the N-nitroso derivative, which was then reduced with zinc to form UDMH, isolated as the salt. Hatt later made UDMH by treating dimethylamine with chloramine; this is one of two current industrial production processes. In the other, acetylhydrazine is treated with formaldehyde and hydrogen to form a dimethyl derivative, which is then hydrolyzed to produce UDMH.
UDMH is a hypergolic rocket fuel, which means that it ignites when it is mixed with another component of the fuel. The second component is usually dinitrogen tetroxide. UDMH is generally preferred to hydrazine in rocket fuels because it autoignites at a higher temperature and is thus more stable in storage.
1. CAS Reg. No. 593-82-8.
1,1-Dimethylhydrazine hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Flammable liquids, category 2 | H225—Highly flammable liquid and vapor | |
| Acute toxicity, oral, category 3 | H301—Toxic if swallowed | |
| Acute toxicity, dermal, category 3 | H311—Toxic in contact with skin | |
| Skin corrosion/irritation, category 1B | H314—Causes severe skin burns and eye damage | |
| Serious eye damage/eye irritation, category 1 | H318—Causes serious eye damage | |
| Acute toxicity, inhalation, category 2 | H331—Fatal if inhaled | |
| Germ cell mutagenicity, category 2 | H340—Suspected of causing genetic defects | |
| Carcinogenicity, category 1B | H350—May cause cancer | |
| Specific target organ toxicity, single exposure, category 1 | H370—Causes damage to respiratory system, nervous system | |
| Specific target organ toxicity, repeated exposure, category 1 | H372—Causes damage through prolonged or repeated exposure to liver, blood system, respiratory system, nervous system | |
| Short-term (acute) aquatic hazard, category 2 | H401—Toxic to aquatic life | |
| Long-term (chronic) aquatic hazard, category 2 | H411—Toxic to aquatic life with long-lasting effects | |
*Compilation of two safety data sheets.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
Molecule of the Future
Darobactin A1 is a potential antibiotic that was discovered in 2019 by Kim Lewis at Northeastern University (Boston) and 14 collaborators there and at several other institutions in the United States and Germany. The authors isolated the molecule from species of Photorhabdus bacteria that live in the digestive systems of certain nematodes.
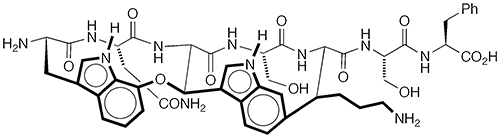
Lewis et al. found that darobactin A cured infections in laboratory animals caused by Gram-negative bacteria in the Enterobacteriaceae family, including Escherichia coli. In 2022, two total syntheses of darobactin A were reported: Niki R. Patel, David A. Petrone, David Sarlah, and collaborators at Merck (Rahway, NJ) and the University of Illinois at Urbana–Champaign performed the synthesis in 16 steps; Phil S. Baran at Scripps Research (La Jolla, CA) used 18 steps to accomplish the feat.
1. CAS Reg. No. 2409072-20-2.
Molecule of the Future
Once a month we bring you a newly discovered or developed molecule that has important implications for the future of chemistry or society in general. Look for it the third week of each month. Learn more about this month's Molecule of the Future below.
We're looking for more molecules of the future!
Do you have a suggestion for the next molecule of the future? Send your idea to MOTW.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
1,1-Dimethylhydrazine
fast facts
| CAS Reg. No. | 57-14-7 |
| SciFinder nomenclature | Hydrazine, 1,1-dimethyl- |
| Empirical formula | C2H8N2 |
| Molar mass | 60.10 g/mol |
| Appearance | Colorless liquid |
| Boiling point | 64 °C |
| Water solubility | Miscible |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

