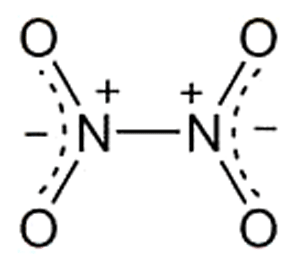
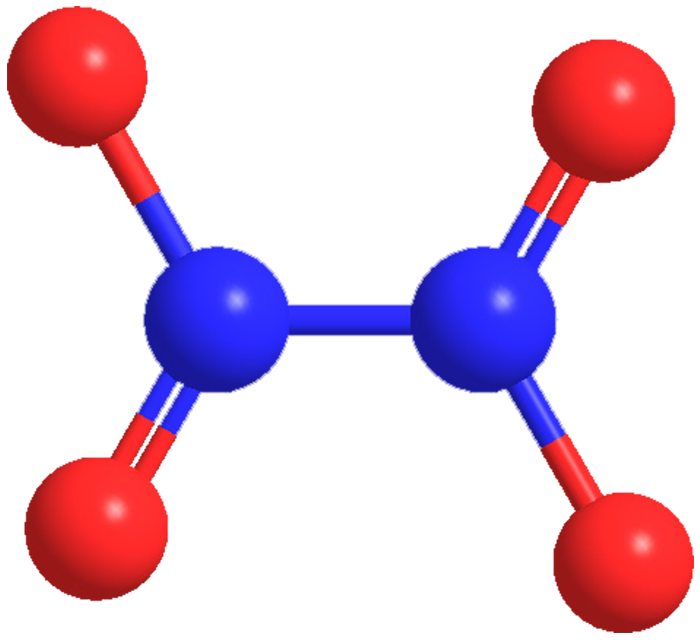
Highly toxic and corrosive dinitrogen tetroxide is in equilibrium with NO2 in its liquid and vapor states. Its brownish color comes from NO2; N2O4 is colorless. It is used commercially as an intermediate in the manufacture of HNO3 and as a nitrating and oxidizing agent. N2O4 is the oxidizer of choice for storable rocket propellants because it is hypergolic with hydrazine fuels.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

