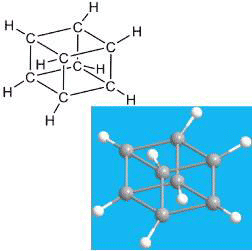
The highly symmetrical C8H8 hydrocarbon cubane was first synthesized by Philip Eaton in 1964. Before that, many thought that it could not be synthesized because of the great strain generated by its 90° C–C–C bond angles. Cubane is isomeric with cyclooctatetraene, and this relationship has been used to synthesize cyclooctatetraenophanes from cubane-derivative building blocks.
MOTW update: January 2, 2023
As its name implies, Cubane1 is a highly symmetrical cubic molecule. It is thermally unstable because of the high strain of its 90° bond angles.
In December, Xujun Cheng, Stephen L. Craig, and co-workers at Duke University (Durham, NC) described the mechanochemistry of cubane. When a cubane derivative with polymeric side chains at the 1- and 2-positions was subjected to pulsed ultrasonication, the only product was the corresponding syn-tricyclooctadiene, in contrast to the cyclooctatetraene derivative formed by thermal isomerization.
1. CAS Reg. No. 277-10-1.
MOTW update:
September 25, 2023
Cubane1 Once thought to be impossible to synthesize because of its high angle strain, the feat was finally accomplished in 1964 by Philip E. Eaton* and Thomas W. Cole, Jr, at the University of Chicago. In 2015, Ronny Priefer at Western New England University (Springfield, MA) and coauthors at other institutions wrote a 50-year review of the molecule.
Last December, Xujun Zheng, Stephen L. Craig, and colleagues at Duke University (Durham, NC) described a study of the mechanochemistry of cubane. In one example, they found that force applied to a 1,2-disubstituted cubane produced a single tricyclic cubane isomer, whereas heating the same derivative led to multiple decomposition products.2
1. CAS Reg. No. 277-10-1.
2. This update was suggested by a reader.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.


