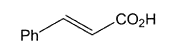
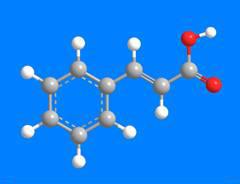
Cinnamic acid exists as trans and cis isomers, but the trans form is the one most often found in nature and is the article of commerce. It is obtained from cinnamon bark and balsam resins such as storax. It was first isolated in 1872 by F. Beilstein (of Handbook of Organic Chemistry fame) and A. Kuhlberg. It is synthesized by the Perkin reaction between Ac2O and PhCHO.
trans-Cinnamic acid is used in the manufacture of flavors, dyes, and pharmaceuticals; but its major use is for the production of its methyl, ethyl, and benzyl esters. These esters are important components of perfumes. The acid is also a precursor to the sweetener aspartame.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

