What molecule am I?

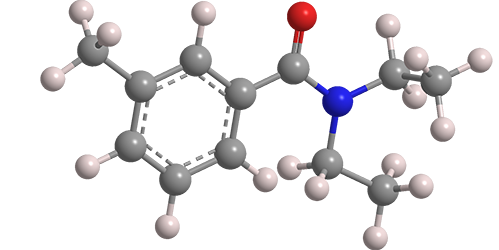
N,N-Diethyl-m-toluamide, known familiarly as DEET, has been the most widely used active ingredient in insect repellents since 1957. The compound was mentioned in the literature under the name “Autan” in connection with disinfection processes as early as 1907, but its first reported synthesis was in 1929 by N. N. Maxim in a Romanian journal.
Maxim’s article did not mention DEET’s use as an insect repellent; but in 1954, E. T. McCabe and colleagues at the US Department of Agriculture (Beltsville, MD) reported the preparation of 33 diethylamides of aromatic acids and concluded: “The most promising mosquito repellents of this series were derived from the ring-substituted benzoic acids.”—including DEET. Samuel Gertler, one of McCabe’s coauthors, had developed the compound 10 years earlier for use by the US Army as a result of soldiers’ experiences in jungle combat during World War II.
DEET has been used effectively and, for the most part, safely for more than 60 years. But, as the hazard information table shows, it can cause some skin problems and has environmental concerns. Only rare cases of DEET toxicity or severe skin reactions have been reported.
As successful as DEET is , an improved product may be on its way to market. See this week’s Molecule of the Future below.
Originally the Molecule of the Week for September 22, 2008
N,N-Diethyl-m-toluamide hazard information
| Hazard class* | GHS code and hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 4 | H302—May be harmful if swallowed | |
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Short-term (acute) aquatic hazard, category 3 | H402—Harmful to aquatic life | |
| Long-term (chronic) aquatic hazard, category 3 | H412—Harmful to aquatic life with long-lasting effects | |
*Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
Molecule of the Future
p-Menthane-3,8-diol1 (PMD), found in the lemon eucalyptus tree (Corymbia citriodora), is the only natural substance recommended by the US Centers for Disease Control and Prevention as a mosquito repellent. It occurs in nature in all four possible diastereomeric forms, which were at one time considered to be equally active against Anopheles gambiae, the main vector for malaria in sub-Saharan Africa.
Marc Lemaire and his research groups at Claude Bernard University Lyon 1 (Villeurbanne, France) and the affiliated International Associated Laboratory (Antananarivo, Madagascar) were skeptical that all four isomers could have the same effect, so they synthesized them from citronellal2 and tested them against an Asian mosquito species, Aedes albopictus, that spreads diseases such as Zika and dengue. They found that under practical conditions, the diastereomer (1R)-(+)-cis-p-menthane-3,8-diol3 performed the best because it is the least volatile and therefore lasts the longest on human skin.
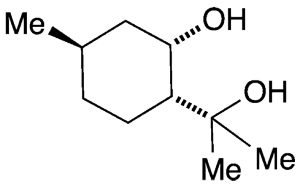
(1R)-(+)-cis-PMD’s repellence duration was similar to that of DEET; and the natural substance is significantly less toxic. The synthesis from commercially available citronellal is relatively straightforward, which makes Lemaire optimistic that the key isomer will be a practical alternative to DEET.
1. CAS Reg. No. 42822-86-6.
2. CAS Reg. No. 106-23-0.
3. CAS Reg. No. 92471-23-3.
Molecule of the Future
Once a month we bring you a newly discovered or developed molecule that has important implications for the future of chemistry or society in general. Look for it the third week of each month. Learn more about this month's Molecule of the Future below.
We're looking for more molecules of the future!
Do you have a suggestion for the next molecule of the future? Send your idea to MOTW.
Molecules from the Journals
MOTW briefly describes noteworthy molecules that appeared in recent ACS journal articles. See this week's
edition below.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
N,N-Diethyl-m-toluamide
fast facts
| CAS Reg. No. | 134-62-3 |
| SciFinder nomenclature | Benzamide, N,N-diethyl-3-methyl- |
| Empirical formula | C12H17NO |
| Molar mass | 191.27 g/mol |
| Appearance | Colorless to light yellow liquid |
| Boiling point | 285–290 °C |
| Water solubility | ≈10 g/L |
Molecules from the journals
Legionaminic acid1 and pseudaminic acid2 are the best-known members of the nine-carbon aminodeoxysialic acids that are found only in bacteria, including some pathogenic species. In April this year, Sameera Siyabalapitiya Arachchige and David Crich* at the University of Georgia (Athens) synthesized these and related molecules from N-acetylneuraminic acid3, the predominant human sialic acid, via side-chain exchange.
Also in April, Yuji Ikeda and co-workers at Stuttgart University (Germany) reported the preparation of Li5Sn4, the most lithium-rich binary stannide to date. This compound has the space group Cmcm, which the authors believe is important for its performance in Li–Sn batteries.
1. CAS Reg. No. 402942-28-3.
2. CAS Reg. No. 1610606-92-2.
3. CAS Reg. No. 131-48-6.
4. CAS Reg. No. 445311-21-7.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

