What molecule am I?
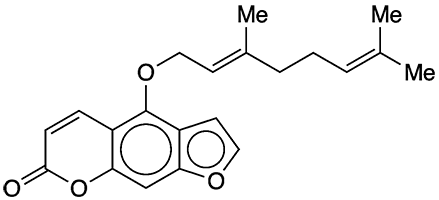
Furanocoumarins are organic compounds found in the seeds and fruits of many species of plants, notably in the families Rutaceae (citrus fruits) and Apiaceae (celery, carrots, and others). As the name suggests, the central structure of furanocoumarins consists of a furan ring fused to a two-ring coumarin system.
Psoralen1 and angelicin2 are unsubstituted furanocoumarins in which the furan and coumarin rings are fused in linear and angular orientations, respectively. Bergamottin and its oxidation products 6′,7′-dihydroxybergamottin3 (DHB) and 6′,7′-epoxybergamottin4 are psoralen derivatives that have geraniol-derived side chains attached at the 5-position. Bergamottin is named after the bergamot orange (Citrus bergamia); it was first isolated from bergamot oil in 1937 by Ernst Späth and Paul Kainrath at the University of Vienna.
Bergamottin plays a role in the lives of many people. It and DHB inhibit the cytochrome P450 enzyme CYP3A, thereby slowing or preventing the metabolism of lovastatin, simvastatin, atorvastatin, and other “statin” drugs that decrease the formation of low-density lipoprotein (LDL) carriers of cholesterol in the body. Bergamottins are particularly abundant in grapefruit, which is why statin labels advise against consumption of the fruit or its juice close to the time the drugs are taken.
The oxidized bergamottins and other grapefruit ingredients also inhibit the transport of talinolol5, a substrate for P glycoprotein, an important cell membrane component that removes foreign substances from cells. For more information on bergamottin and its derivatives, see this Science Direct entry.
1. CAS Reg. No. 66-97-7.
2. CAS Reg. No. 523-50-2.
3. CAS Reg. No. 145414-76-2.
4. CAS Reg. No. 206978-14-5.
5. CAS Reg. No.57460-41-0.
Bergamottin hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Skin sensitization, category 1 | H317—May cause an allergic skin reaction | |
*Most safety data sheets describe bergamottin as not a hazardous substance or mixture.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
Molecules from the journals
Cobalt(II) bromide1 (CoBr2) is a green crystalline solid that was known as long ago as 1883. In 1922, George L. Clark and Henry K. Buckner at the University of Chicago prepared it and its hydrates from cobalt carbonate and hydrobromic acid. Although its anhydrous form is green, its hydrates range in color from pink to blue.
CoBr2 has a long history as a catalyst for organic chemical reactions. This year, Grégory Danoun and colleagues at the Polytechnic Institute of Paris (Paliseau, France) reported that it is the only catalyst needed for simple, straightforward Negishi-type cross-couplings of aryl amides to produce alkyl aryl ketones.
(−)-Phaeocaulisin A2 is a guaiane-type sesquiterpene found in the Chinese ginger plant Curcuma phaeocaulis. The plant has long been used in traditional medicine; but in 2013, researchers began to study the isolated molecule for potential drug development. This year, Áron Péter, Giacomo E. M. Crisenza, and David J. Procter* at the University of Manchester (UK) reported an enantioselective total synthesis of (−)-phaeocaulisin A in 17 steps with 2% overall yield from commercially available starting materials and reagents.
1. CAS Reg. No. 7789-43-7.
2. CAS Reg. No. 1443004-41-8.
Molecules from the Journals
MOTW briefly describes noteworthy molecules that appeared in recent ACS journal articles. See this week's
edition below.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Bergamottin fast facts
| CAS Reg. No. | 7380-40-7 |
| SciFinder nomenclature | 7H-Furo[3,2-g][1]benzopyran-7-one, 4-{[(2E)-3,7-dimethyl-2,6-octadien-1-yl]oxy}- |
| Empirical formula | C21H22O4 |
| Molar mass | 338.40 g/mol |
| Appearance | White crystals or powder |
| Melting point | 55–56 °Ca |
| Water solubility | ≈10 mg/L |
a. Some sources report 59–60 °C or 75–80 °C.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

