What molecule am I?
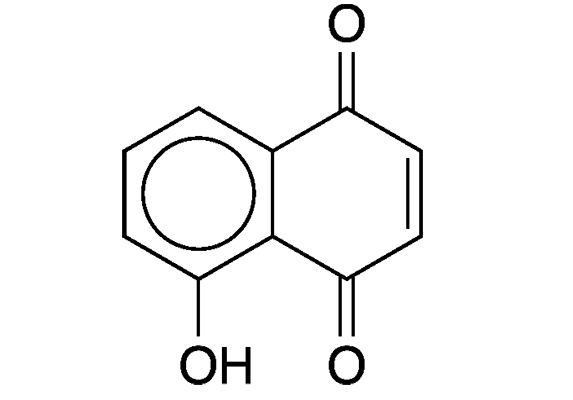
Juglone (5-hydroxy-1,4-naphthalenedione) is a naturally occurring naphthoquinone derivative that has been known for more than a century. In 1905, French scientists M. Brissemoret and R. Combes reported that juglone exists in the green portions of walnut trees (leaves, stems, and husks). Their report cites articles from 1884. In 1887, German chemists August Bernthsen and August Semper described juglone’s structure and its synthesis from naphthalene.
Juglone is found in many species of the walnut family (Juglandaceae), particularly in eastern American black walnut (Juglans nigra). The compound serves as a defense mechanism against surrounding plants: It can stunt their growth, a process known as allelopathy. Since its discovery, juglone has occasionally been used as an herbicide and an anthelmintic (a group of antiparasitic drugs). It is also ichthyotoxic, and some cultures catch fish by throwing walnut husks into the water.
Juglone is more frequently used as a dye. In its pure form, it has a yellow color; but when it is exposed to oxygen, it turns orange, red, or, mainly, brown. As a dye, it is known as C.I. Natural Brown 7, a classification of the database Colour Index International. It is used to dye fabrics, cosmetics, and even foods, despite its properties listed in the hazard information table. It is chemically related to henna.
To learn how the naphthalene-like structure of juglone is biosynthesized, read the 2018 Horticulture Research article by Joshua R. Widhalm and co-workers at Purdue University.
Juglone hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 3 | H301—Toxic if swallowed | |
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
| Specific target organ toxicity, single exposure, narcotic effects, category 3 | H336—May cause drowsiness or dizziness | |
*Compilation of multiple safety data sheets.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
Molecules from the journals
Rucaparib1 is a poly(ADP-ribose) polymerase (PARP) inhibitor that combats cancer. In 2016, the US Food and Drug Administration approved its use for treating ovarian cancer; and in 2020, it was approved for treating prostate cancer. This March, Jinjae Park and Cheol-Hong Cheon* at Korea University (Seoul) reported a three-step total synthesis of rucaparib from commercially available starting materials in 54% overall yield.
Bromonitromethane2 (BrCH2NO2), a C1 molecule with two functional groups, has been known since 1915. It is a versatile reagent that is used to synthesize nitrogen heterocycles, nitrocyclopropanes, and enantiopure amines, amides, and peptides. Recently, BrCH2NO2 has been in short supply, compelling Jeffrey N. Johnston and colleagues at Vanderbilt University (Nashville) to develop a large-scale synthesis of the reagent. They optimized existing methods to create a concise procedure in which nitromethane is treated with aqueous sodium hydroxide and sodium bromide, followed by bromine addition, to produce BrCH2NO2 in >70% yield with minimal formation of the dibromonitromethane side product.
1. CAS Reg. No. 283173-50-2.
2. CAS Reg. No. 563-70-2.
Molecules from the Journals
MOTW briefly describes noteworthy molecules that appeared in recent ACS journal articles. See this week's
edition below.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Juglone fast facts
| CAS Reg. No. | 481-39-0 |
| SciFinder nomenclature | 1,4-Naphthalenedione, 5-hydroxy- |
| Empirical formula | C10H6O3 |
| Molar mass | 174.15 g/mol |
| Appearance | Yellow to brown crystals or powder |
| Melting point | 161–163 °C |
| Water solubility | 2–5 g/L (est.) |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

