What molecule am I?

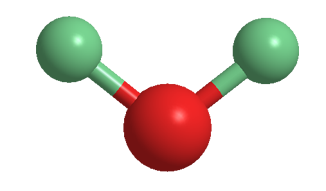
Deuterium oxide (D2O), aka “heavy water”, is the form of water that contains two atoms of the 2H, or D, isotope. The term heavy water is also used for water in which 2H atoms replace only some of the 1H atoms. In this case, rapid exchange between the two isotopes forms twice as many “semiheavy” HDO molecules as D2O.
Harold Urey, the 1934 Nobel Prize laureate in chemistry, pioneered deuterium chemistry. In 1931, he and his colleagues at Columbia University (New York City) carefully distilled 5 L of liquid nitrogen to produce 1 mL of molecular deuterium. Shortly afterward, they produced D2O from ordinary water by using prolonged electrolysis.
For decades, D2O has been extremely useful in many chemical applications. The difference between a reaction rate in D2O solvent versus that in H2O often provides clues as to the reaction’s mechanism. This is especially important if water is one of the reactants.
In some nuclear reactors, D2O is used to slow down neutrons so that they react with fissionable 235U rather than nonfissioning 238U, thus eliminating the need for uranium enrichment. D2O is superior to H2O for this use because of its ≈6 times greater thermal neutron capture cross section.
Deuterium oxide hazard information
| GHS classification*: not a hazardous substance or mixture |
*Globally Harmonized System of Classification and Labeling of Chemicals. Explanation of pictograms.
Deuterium oxide fast facts
| CAS Reg. No. | 7789-20-0 |
| Empirical formula | D2O |
| Molar mass | 20.03 g/mol |
| Appearance | Colorless liquid |
| Melting point | 3.8 ºC |
| Boiling point | 101.4 ºC |
| Water solubility | Miscible |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

