What molecule am I?
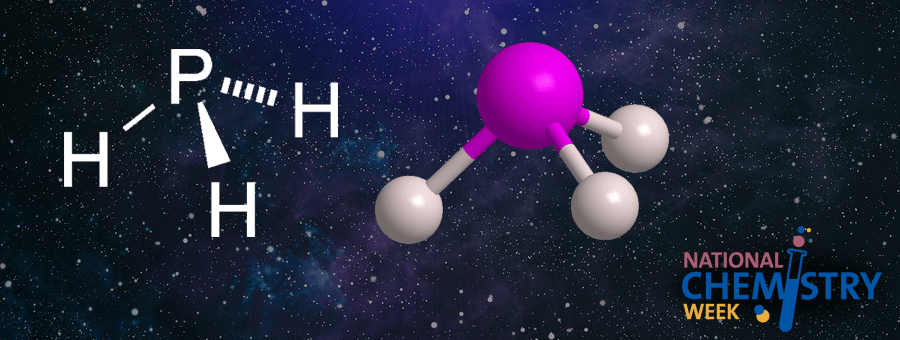
Phosphine, the simplest phosphorus hydride, is a colorless and extremely toxic gas. Some people think it smells like rotting fish; it reminds others of the odor of garlic. In any case, pure phosphine is actually odorless; an impurity, diphosphane (P2H4), is responsible for its foul scent.
Phosphine is formed naturally via the anaerobic decay of phosphorus-containing organic matter. The earliest reported preparation was made by Antoine Lavoisier’s student Philippe Gengembre in the late 18th century.
Phosphine is made industrially from white phosphorus by hydrolysis with an alkali metal hydroxide or an aqueous acid–catalyzed disproportionation reaction. The industrial product is normally shipped as liquefied gas.
Although phosphine’s molecular structure is analogous to that of ammonia, the 3d orbital of its phosphorus atom interacts with its hydrogen atoms to reduce its ability to hydrogen bond. Thus, whereas ammonia is completely miscible in water, the aqueous solubility of phosphine is very low.
The hazard information box shows that phosphine is dangerous in several ways on Earth. But recent findings indicate that its presence in outer space may have had a key role in the beginnings of life.
Ralf I. Kaiser at the University of Hawaii at Manoa (Honolulu) and his colleagues there and in Taiwan and France simulated ices found in space by combining phosphine, carbon dioxide, water, and oxygen at near–absolute zero temperatures. They then bombarded the ices with high-energy electrons (typical of those found in space) to produce several phosphorus oxyacids, including some with the same phosphorus oxidation state that exists in DNA, ATP, and other important biomolecules.
The theme of this year’s National Chemistry Week (which begins today) is “Chemistry Is Out of This World”. Celebrate it by recognizing extraterrestrial phosphine’s potential contribution to life.
Phosphine hazard information
| GHS classification*: flammable gases, category 1 | |
| H220—Extremely flammable gas | |
| GHS classification: gases under pressure, compressed gas | |
| H280—Contains gas under pressure; may explode if heated | |
| GHS classification: skin corrosion, category 1B | |
| H314—Causes severe skin burns and eye damage | |
| GHS classification: serious eye damage, category 1 | |
| H318—Causes serious eye damage | |
| GHS classification: acute toxicity, inhalation, category 1 | |
| H330—Fatal if inhaled | |
| GHS classification: hazardous to the aquatic environment, acute hazard, category 1 | |
| H400—Very toxic to aquatic life | |
*Globally Harmonized System of Classification and Labeling of Chemicals. Explanation of pictograms.
Phosphine fast facts
| CAS Reg. No. | 7803-51-2 |
| Empirical formula | H3P |
| Molar mass | 34.00 g/mol |
| Appearance | Colorless gas |
| Boiling point | –87.7 ºC |
| Water solubility | ≈300 mg/L |
MOTW update: September 28, 2020
Phosphine is a toxic gas that is used in industrial synthesis. In recent years, phosphine was detected in outer space; some scientists suggest that it may be the source of phosphorus in biomoecules. Just in the past week, Jane S. Greaves at Cardiff University and the University of Cambridge (UK) and several colleagues worldwide reported the discovery of phosphine in the clouds that cover the planet Venus. This truly exciting finding was described in detail in major news outlets because of the possible presence of life on the shrouded planet.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

