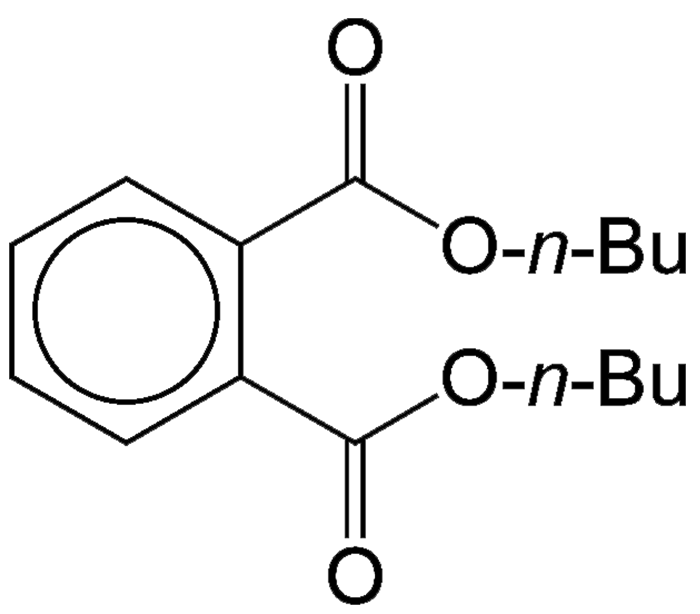
Di-n-butyl phthalate (DBP) is an oily, sparingly water-soluble liquid with a very high boiling point (350 ºC). In 1962, R. H. Mills, M. W. Farrar, and O. J. Weinkauff at Monsanto reported its preparation from phthalic acid and n-butyl chloride. More recently, the predominant manufacturing method was the reaction of phthalic anhydride with n-butyl alcohol.
DBP’s original use was as an insect repellent that was impregnated into clothing; but it gained its greatest use (and notoriety) as a plasticizer for poly(vinyl chloride) used in many consumer products. In the 1980s, toxicological studies revealed it to be a suspected endocrine disruptor and teratogen; and by the late 2000s, it had been banned for all uses in the United States and the European Union.
Some chemical companies, including producers of phthalate esters, are now marketing phthalate replacements. Examples are Eastman’s Benzoflex (benzoate ester) and Effusion (di-n-butyl terephthalate) lines of PVC plasticizers.
MOTW Update:
February 13, 2017
Bis(2-ethylhexyl) phthalate, di-n-butyl phthalate, methylparaben, and hexabromocyclododecane are all former Molecules of the Week. Last month, Janet Pelley wrote in ACS Central Science that these and many other commercial products are principal components of common household dust. (A similar article appears in this week’s C&EN.) The alarming aspect of these findings is that all of these compounds are toxic, primarily to the human reproductive, hormonal, and nervous systems.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

