
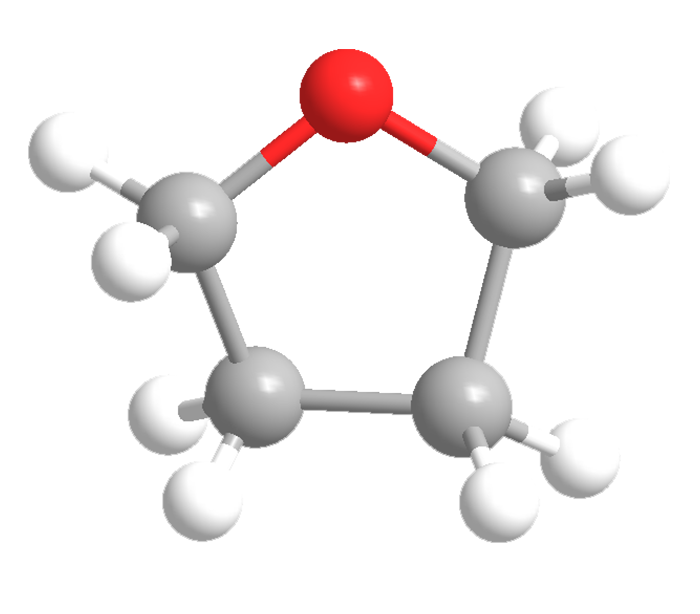
Tetrahydrofuran (THF, oxolane) is a versatile solvent used in laboratory organic synthesis and in industrial products such as varnishes. It is colorless and miscible in water, with a boiling point of 66 ºC. It is highly flammable but relatively nontoxic. When stored in air, it can form explosive peroxides, but this can be prevented by adding an inhibitor such as butylated hydroxytoluene (BHT).
In 1956, W. W. Gilbert and B. W. Howk at Du Pont patented the catalytic hydrogenation of maleic anhydride to produce THF. Du Pont later patented a process for hydrogenating furan to THF. Today the predominant THF manufacturing process is the acid-catalyzed dehydration of 1,4-butanediol. More than two dozen US patents on this process were issued between 1975 and 2007.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

