What molecule am I?
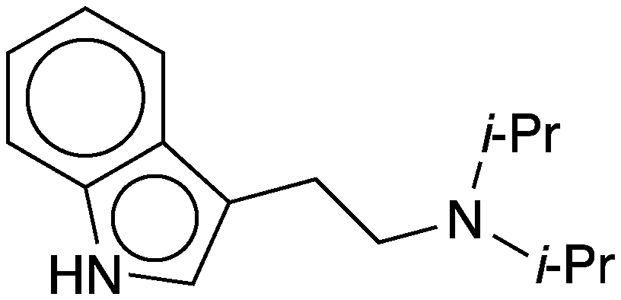
N,N-Diisopropyltryptamine (commonly abbreviated as DiPT) is a synthetic hallucinogenic substance. It is a derivative of the amino acid tryptophan and is closely related to the naturally occurring and widely banned N,N-dimethyltryptamine1 (DMT), the Molecule of the Week for September 17, 2018.
American biochemist Alexander T. Shulgin, the so-called “godfather of psychedelics”, synthesized and experimented with DiPT as early as 1980. His 1997 book TIHKAL2: The Continuation, coauthored with his wife Ann, described their research, including their own experiences, with DiPT and other tryptamine derivatives.
Shulgin published two methods for synthesizing DiPT as its hydrochloride. In the first, he combined indole with oxalyl chloride to form an acyl chloride intermediate, which was treated with diisopropylamine to form indol-3-yl-N,N-diisopropylglyoxylamide. This product was then reduced with lithium aluminum hydride to form DiPT∙HCl.
In the second, more direct, synthesis, Shulgin treated tryptamine with isopropyl iodide in the presence of a tertiary amine. The crude free base DiPT was acidified with anhydrous hydrogen chloride to form the HCl salt.
The same article goes on to describe DiPT’s hallucinogenic effects. DiPT is unlike all other psychedelic substances in that it produces primarily auditory effects. At moderate to high doses, it lowers the pitch of perceived sounds. In Shulgin’s experience when he ingested 25 mg:
My wife’s voice is basso, as if she had a cold—my ears with slight pressure as if my tubes were clogged but they aren’t. Radio voices are all low, music out of key. Piano sounds like a bar-room disaster. The telephone ringing sounds partly underwater. In a couple more hours, music pretty much normal again.
DiPT is not a scheduled controlled substance in the United States, although its 5-methoxy derivative (aka “foxy methoxy”) is illegal. Possession of DiPT, however, could possibly be prosecuted under the Federal Analogue Act.
1. DMT continues to be studied as a replacement for side effect–prone ketamine for treating severe depression
2. Abbreviation for “Tryptamines I Have Known and Loved”.
N,N-Diisopropyltryptamine hazard information
| Hazard class* | Hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 4 | H302—Harmful if swallowed | |
| Acute toxicity, dermal, category 4 | H312—Harmful in contact with skin | |
| Acute toxicity, inhalation, category 4 | H332—Harmful if inhaled | |
*Globally Harmonized System of Classification and Labeling of Chemicals.
Explanation of pictograms.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
N,N-Diisopropyltryptamine
fast facts
| CAS Reg. No. | 14780-24-6 |
| SciFinder nomenclature | 1H-Indole-3-ethanamine, N,N-bis(1-methylethyl)- |
| Empirical formula | C16H24N2 |
| Molar mass | 244.38 g/mol |
| Appearance | White crystalline solid |
| Melting point | 46 ºC |
| Water solubility | ≈14 g/L (est) |
MOTW update
Titanium dioxide (TiO2) was the Molecule of the Week for June 27, 2005. For decades it has been widely used for its brilliant, reflective whiteness in applications ranging from paints to sunscreens. Although it has long been thought to be safe as a food and toothpaste ingredient, recent studies revealed it to be a possible carcinogen. This month, a panel of the European Food Safety Authority reported that TiO2 could not be ruled out as a genotoxin. It is expected that the European Union will soon ban it as a food additive.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

