What molecule am I?

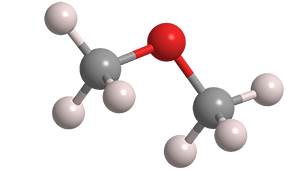
Dimethyl ether (DME), a colorless gas, is the simplest aliphatic ether. Also called methyl ether, it is currently produced by dehydrating methanol; but researchers have an eye on it for sustainable production from biogenic methane or biomass derived from cellulose or lignin.
DME is primarily used to make other small molecules such as acetic acid or dimethyl sulfate. It is also a spray-can propellant1 and a refrigerant to replace chlorofluorocarbons. It has been proposed as a cleaner-burning fuel than hydrocarbons.
Far beyond its terrestrial presence, DME was a significant recent discovery in outer space. In March, researchers at Leiden University (the Netherlands) used the Atacama Large Millimeter/submillimeter Array telescope to identify the molecule in a planet-forming disc in the constellation Ophiuchus, 444 light-years from Earth. With nine atoms, DME is the largest molecule yet observed in space.
Always optimistic, some astrobiologists have speculated that the presence of DME could be an indicator of life “out there”.
1. Erik Rotheim, the Norwegian inventor of the spray can, cited the use of DME in one of his patents, US 1,800,156, in 1931.
Dimethyl ether hazard information
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Flammable gases, category 1 | H220—Extremely flammable gas | |
| Gases under pressure, liquefied gas | H280—Contains gas under pressure; may explode if heated | |
| Simple asphyxiant, category 1*** | May displace oxygen and cause rapid suffocation | |
| Specific target organ toxicity, single exposure; narcotic effects, category 3 | H336—May cause drowsiness or dizziness | |
*Compilation of multiple safety data sheets.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
***Hazard class not included in GHS; no pictogram assigned.
Molecules from the journals
Iodic acid1 (HIO3) is a white, water-soluble solid with a melting point of 110 °C. It is a relatively strong acid (pKa 7.5) and a powerful oxidizer. One of its main uses is the preparation of iodate salts for use in “iodized” table salt.
This past May, Juan Carlos Gómez Martín, Alfonso Saiz-Lopez, and colleagues at the Institute of Astrophysics of Andalusia (Granada, Spain), the Institute of Physical Chemistry Rocasolano (Madrid), and the University of Leeds (UK) described the role of iodic acid in new-particle formation in marine and polar boundary layers. Particle formation is suspected of accelerating sea ice melting.
Pleuromutilin2 is an antibacterial terpenoid isolated from fungi of the former genus Pleurotus (now Clitopilus) in 1951 by Frederick Kavanagh, Annette Hervey, and William J. Robbins at Columbia University (New York City). Several of its derivatives are the active ingredients in current antibiotics such as lefamulin3 and retapamulin4.
Four research groups reported the total synthesis of pleuromutilin between 1982 to 2017. Just last month, Nicholas J. Foy and Sergey V. Pronin* at the University of California, Irvine, described an improved fifth synthesis. They accomplished the feat in 16 reaction steps from commercially available materials.
1. CAS Reg. No. 7782-68-5.
2. CAS Reg. No. 125-65-5.
3. CAS Reg. No. 1061337-51-6.
4. CAS Reg. No. 224452-66-8.
Molecules from the Journals
MOTW briefly describes noteworthy molecules that appeared in recent ACS journal articles. See this week's
edition below.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Dimethyl ether fast facts
| CAS Reg. No. | 115-10-6 |
| SciFinder nomenclature | Methane, 1,1′-oxybis- |
| Empirical formula | C2H6O |
| Molar mass | 46.07 g/mol |
| Appearance | Colorless gas |
| Boiling point | –25 °C |
| Water solubility | 71 g/L (STP)a |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

