What molecule am I?
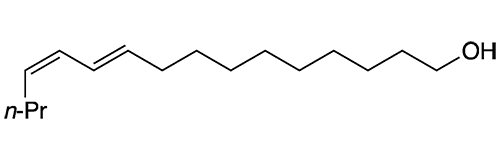
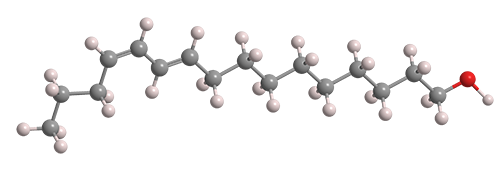
Pheromones—hormonal substances that animals and some other organisms emit to attract or warn other members of their species—are well known today. But it has been only ≈60 years since they were first discovered and characterized. Bombykol, a sex pheromone released by female silkworm moths such as Bombyx mori and B. mandarina, was the first one to be studied thoroughly.
The pioneering work on bombykol was done by Adolf Butenandt and his co-workers at the Max Planck Institute of Biochemistry (Munich). He named the molecule after the silkworm genus, determined its structure, synthesized it, and demonstrated that its effects duplicated those of the natural product. Twenty years before his work on bombykol, he was awarded the 1939 Nobel Prize in Chemistry for his research on other sex hormones.1
From Butenandt’s time until now, much research has been done on the biochemistry of bombykol:
- In 1987, Tetsu Ando and colleagues at the Tokyo University of Agriculture and Technology elucidated its biosynthetic pathway.
- In 1995, Shogo Matsumoto and co-workers at the Institute of Physical and Chemical Research (RIKEN) (Saitama, Japan) reported a biosynthesis of the compound outside of silkworm cells.
- In 1999, Jon Clardy and coauthors at Cornell University (Ithaca, NY) and the National Institute of Sericultural and Entomological Sciences (Tsukuba Science City, Japan) determined the structure of the protein in the male B. mori that binds bombykol emitted by the female.
Finally, in 2020, Jean-François Dufrêche and collaborators at the University of Montpellier Marcoule (Bagnols-sur-Cèze, France) and CNRS–University of Tours (France) proposed a theory for how pheromone molecules are transported in an atmosphere that contains aerosols—even though many pheromones are hydrophobic. They used bombykol as a model compound in their study.
1. Butenandt was affiliated with the Nazi party, which damaged his reputation in the years after World War II.
Bombykol hazard information
| Hazard class* | GHS code and hazard statement |
|---|---|
| Not a hazardous substance or mixture |
*Globally Harmonized System (GHS) of Classification and Labeling of Chemicals.
MOTW update
(–)-Strychnine1 was the Molecule of the Week for December 2, 2013. It is a virulent poison produced in the seeds of plants of the Strychnos genus. Its non-natural enantiomer, (+)-strychnine2, was first synthesized in 1995.
In the 1940s, Robert Robinson (Oxford University) and Robert B. Woodward (Harvard University), both eventual Nobel laureates in chemistry, published structures of the natural strychnine molecule. Since then, there has been controversy as to which chemist should be credited for first describing the true structure.
In a recent survey of chemists conducted by Jeffrey I. Seeman* at the University of Richmond (VA) and Mark C. House at Santa Fe College (Gainesville, FL), the respondents did not reach a consensus as to which Nobelist deserves the credit. The authors concluded that
1. CAS Reg. No. 57-24-9.
2. CAS Reg. No. 163956-38-5.
Molecules from the Journals
MOTW briefly describes noteworthy molecules that appeared in recent ACS journal articles. See this week's
edition below.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Bombykol fast facts
| CAS Reg. No. | 765-17-3 |
| SciFinder nomenclature | 10,12-Hexadecadien-1-ol, (10E,12Z)- |
| Empirical formula | C16H30O |
| Molar mass | 238.41 g/mol |
| Appearance | Colorless to light yellow liquid |
| Boiling point | 130–133 °C (0.005 Torr) |
| Water solubility | ≈0.2 mg/L (est.) |
Molecules from the journals
Ritonavir1 is an older antiretroviral drug developed at Abbott Laboratories (North Chicago, IL) in 1990 and approved by the US Food and Drug Administration in 1996. Nirmatrelvir2 is much newer; it was developed in 2021by Pfizer (New York City) from a previous drug candidate. Later that year, FDA granted Pfizer an emergency use authorization for a combination of the two drugs for treating COVID-19 under the trade name Paxlovid. Read how Pfizer scientists developed Paxlovid in an extraordinarily brief time.
This past May, Deyin Wang, Yuhua Wang, and co-workers at Lanzhou University (China). reported a rapid microwave-radiation synthesis of the complex salt Cs3MnBr53, a blue-light–excitable, narrow-band, green-emitting phosphor. The phosphor had been made previously, but the authors believe that their convenient synthesis will make it a prime candidate for backlighting in white-light–emitting diode displays.
1. CAS Reg. No. 155213-67-5.
2. CAS Reg. No. 2628280-40-8.
3. CAS Reg. No. 2768353-64-4.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

