What molecule am I?
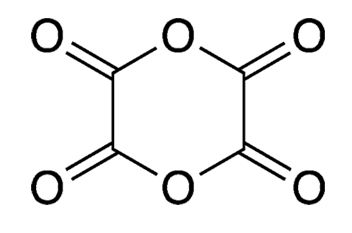
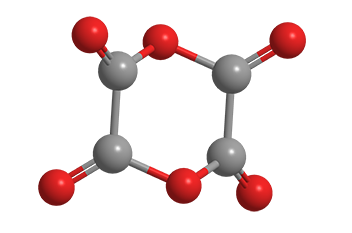
Dioxane tetraketone (C4O6) is a chemical curiosity. Also known as dimeric oxalic anhydride, it’s an oxide of carbon; but unlike carbon monoxide and carbon dioxide, it’s difficult to make and impossible to isolate.
Paolo Strazzolini and colleagues at the University of Udine (Italy) and the University of Zagreb (Croatia) synthesized C4O6 in 1998. They combined oxalyl chloride (or bromide) with a suspension of silver oxalate in diethyl ether at –15 to –10 ºC for 1 h, then, also at low temperature, filtered the unreacted silver salt and evaporated the unreacted dihalide and solvent to leave a white solid.
The authors redissolved the product in CDCl3–Et2O and obtained a 13C NMR spectrum that revealed a single peak (other than the ether peaks) at 144.94 ppm. They concluded that the only plausible interpretation of the spectrum was C4O6, which has a calculated peak of 154 ppm.
C4O6 is stable in ether solution at –30 ºC. When the solution is warmed to 0 ºC, the compound decomposes to CO and CO2. Treating C4O6 with methanol gives the mono- and dimethyl esters of oxalic acid. With diazomethane, the products are dimethyl oxalate and its mono- and bis(diazo) derivatives.
More than two dozen other carbon oxides, ranging from CO3 to C12O12, and with varying degrees of stability, have been reported.
Dioxane tetraketone
fast facts
| CAS Reg. No. | 213967-57-8 |
| Empirical formula | C4O6 |
| Molar mass | 144.04 g/mol |
| Appearance | White solid |
| Melting point | ≈0 ºC (dec.) |
| Water solubility | Reacts |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

