What molecule am I?
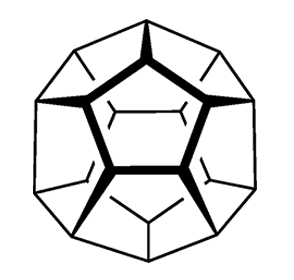
Circa 360 BCE, Plato wrote extensively about the five convex regular polyhedra: the tetrahedron, cube, octahedron, dodecahedron, and icosahedron, with 4, 6, 8, 12, and 20 faces, respectively. He associated four of them with the classical elements earth, air, water, and fire. The shapes came to be known as the platonic solids.
Synthetic organic chemists, being naturally curious, long sought to make hydrocarbons in the shapes of the platonic solids. Examination of the bonding structures and bond angles required for the polyhedra immediately rules out octahedrane (excessively large angle strain) and icosahedrane (requirement for nonexistent pentavalent carbon).
Two of the remaining three platonic hydrocarbons also have substantial angle strain. Tetrahedrane is thought to be kinetically stable, but it has not yet been synthesized. Several tetrahedrane derivatives, however, have been made, beginning with tetra-tert-butyltetrahedrane in 1978 (Rudolf Matusch et al., University of Marburg, Germany). Cubane is also highly strained; but in 1964, Philip E. Eaton* and Thomas W. Cole, Jr., at the University of Chicago reported the synthesis of the unsubstituted hydrocarbon.
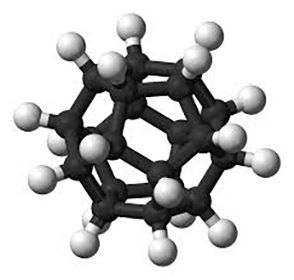
These accomplishments left dodecahedrane, the least strained but most complex platonic hydrocarbon, as the only one not yet synthesized. It was not for lack of trying; for decades, chemists competed to be the first to make it. In 1981, Leo A. Paquette and co-workers at Ohio State University (Columbus) reported the synthesis of 1,16-dimethyldodecahedrane. The next year, the same research group published a synthesis of the parent compound in 23 steps from the cyclopentadienide anion.1
Later in 1982, Paquette wrote a review of what he called “the chemical transliteration of Plato's universe”, which culminated in a detailed account of the dodecahedrane synthesis. Six years later, he and his colleagues published an improved route to the polyhedrane.
With its icosahedral (Ih) symmetry, dodecahedrane is the most highly symmetric hydrocarbon possible. It is in the same symmetry group as the all-carbon buckminsterfullerene. Largely because of its high symmetry, it has an extremely high melting point for a hydrocarbon. Dodecahedrane crystals take the face-centered cubic structure.
1. This was a busy time for Paquette’s group. The subsequent article in that issue of JACS describes their total synthesis of (±)-pentalenene, a complex sesquiterpene and potential antibiotic against Gram-positive and -negative bacteria and pathogenic fungi.
Dodecahedrane hazard information
| Hazard class | Hazard statement |
|---|---|
| No hazard information available | Most likely not a hazardous substance |
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Dodecahedrane
fast facts
| CAS Reg. No. | 4493-23-6 |
| SciFinder nomenclature | [5]Fullerane-C20-Ih |
| Empirical formula | C20H20 |
| Molar mass | 260.37 g/mol |
| Appearance | White crystals |
| Melting point | >450 ºC |
| Water solubility | Insoluble |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

