What molecule am I?
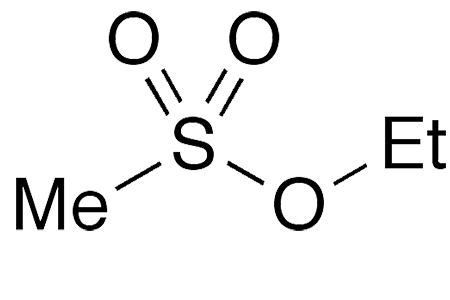
Ethyl methanesulfonate (EMS), also called ethyl mesylate, is a simple, but hazardous, sulfonic acid ester. Its earliest preparation, reported by German organic chemist O. C. Billeter in 1905, was the reaction of methanesulfonic anhydride with ethyl alcohol.
As shown in the hazard information table, EMS is suspected of being mutagenic, teratogenic, and carcinogenic. In an early mutagenicity study (1965), Thomas Alderson at the University of Cambridge (UK) used EMS to induce mutations in fruit flies (Drosophila spp.).
In 1984, Gary A. Sega at Oak Ridge National Laboratory (TN) published an extensive review of EMS’s genetic effects. And last year, in its 15th Report on Carcinogens, the National Toxicology Program (NTP) of the US Department of Health and Human Services included a data sheet on the carcinogenicity of EMS. In addition, NTP also reported a summary of the testing status of the compound.
Because of its extreme toxicity, EMS is produced only for research purposes. Its primary use is as a model alkylating agent in biochemical and medical research, specifically in studies of DNA repair processes.
Molecules from the journals
3-Chlorobiphenyl1 (3-CB) was synthesized in 1924 by Moses Gomberg* and Werner E. Bachmann at the University of Michigan (Ann Arbor) as part of a series of unsymmetrical biaryl molecules made by the reaction of an aryldiazonium salt with another aromatic compound. 3-CB was used in lubricants and hydraulic and heat-transfer fluids; its production and sale were discontinued in 1977.
3-CB, along with other chlorinated and polychlorinated biphenyls (PCBs), is now considered an environmental pollutant. This year, Hans-Joachim Lehmler at the University of Iowa (Iowa City) and colleagues in the United States, China, and Sweden investigated the metabolism of 3-CB in a human liver cancer cell line and identified 20 metabolites, including five that had been dechlorinated.
Astaxanthin2 is a symmetrical long-chain polyolefin with two ketone and two hydroxyl groups. It is a keto-carotenoid that is found in a wide range of animal and plant tissues. In 1938, Richard Kuhn*3 and N. A. Sörensen at the University of Heidelberg (Germany) isolated it from lobster eggs and determined its structure. Astaxanthin is named after the lobster genus Astacus; it is released when lobsters are cooked and is responsible for their red coloration.
Astaxanthin is used as dietary supplement and food dye. According to Wentao Su and co-workers at Dalian Polytechnic University (China), this fat-soluble carotenoid “shows excellent antioxidant and anti-inflammatory activities, but its low biocompatibility and stability limit its application in the food industry.” Accordingly, the researchers devised a hyaluronic acid–modified milk exosome–based astaxanthin delivery system that can be used for efficient inflammatory cell-targeting.
2,5-Dimethyltetrahydrofuran4 (2,5-DMTHF) is a saturated furan derivative that first appeared in the chemical literature in 1907 when Paul Sabatier*5 and A. Mailhe at the University of Toulouse (France) produced it by hydrogenating acetonylacetone (2,5-hexanedione) in the presence of metallic nickel.
Until recently, 2,5-DMTHF received little attention in the scientific world except for a few uses as a cosolvent in battery electrolytes and other electrochemical systems. But because of the safety and environmental disadvantages of traditional aprotic polar solvents, some researchers have begun to take notice. Last month, Weiran Yang, Jing Zheng, and co-workers at Nanchang University (China) reported that sustainable 2,5-DMTHF, manufactured from cellulose, is superior to older solvents such as tetrahydrofuran and 2-methyltetrahydrofuran because of its higher boiling point, lower water solubility, lower vapor pressure, higher flash point, and better thermal and photochemical stability.
1. CAS Reg. No. 2051-61-8.
2. CAS Reg. No. 472-61-7.
3. Kuhn was awarded the 1938 Nobel Prize in Chemistry for his work on carotenoids and vitamins. He was a Nazi collaborator and posthumously censured by the Society of German Chemists.
4. CAS Reg. No. 1003-38-9.
5. Sabatier was awarded the 1912 Nobel Prize in Chemistry along with famed chemist Victor Grignard.
Molecules from the Journals
MOTW briefly describes noteworthy molecules that appeared in recent ACS journal articles. See this week's
edition below.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Ethyl methanesulfonate
fast facts
| CAS Reg. No. | 62-50-0 |
| SciFinder nomenclature | Methanesulfonic acid, ethyl ester |
| Empirical formula | C3H8O3S |
| Molar mass | 124.16 g/mol |
| Appearance | Colorless liquid |
| Boiling point | 213 °C |
| Water solubility | Miscible; hydrolyzes |
Ethyl methanesulfonate hazard information
| Hazard class* | GHS code and hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 4 | H302—Harmful if swallowed | |
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Skin sensitization, category 1 | H317—May cause an allergic skin reaction | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
| Germ cell mutagenicity, category 1B | H340—May cause genetic defects | |
| Carcinogenicity, category 1B | H350—May cause cancer | |
| Reproductive toxicity, category 2 | H361—Suspected of damaging fertility or the unborn child | |
*Globally Harmonized System of Classification and Labeling of Chemicals. Explanation of pictograms.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

