What molecule am I?
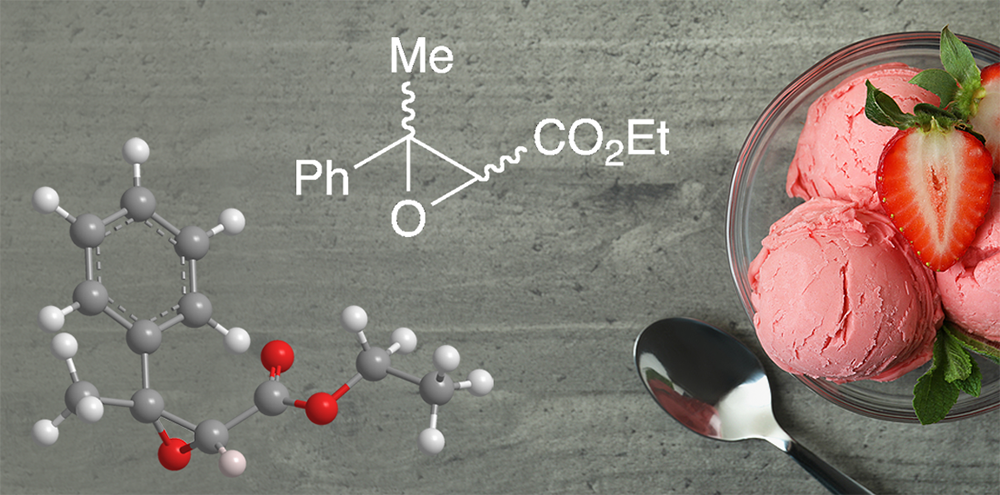
Ethyl methylphenylglycidate is an aromatic organic compound with two functional groups, an ester and an epoxide. It is also known as “strawberry aldehyde” even though it is not found in strawberries and does not contain an aldehyde group. The compound contains two asymmetric centers; the article of commerce is a racemate of all four stereoisomers.
The first known synthesis of ethyl methylphenylglycidate was reported in 1905 by Rainer Ludwig Claisen at the University of Berlin in a lengthy article titled “About some syntheses using sodium amide”. Claisen was a pioneering chemist primarily known for developing condensation and rearrangement reactions that bear his name.
Today, ethyl methylphenylglycidate is synthesized by a method reported in 1961 by Keiiti Sisido, Osamu Nakanisi, and Hitosi Nozaki* at Kyoto University (Japan). They used the Darzens condensation, in which a ketone (acetophenone in this case) is treated with an α-haloester (ethyl chloroacetate) to form the epoxide ring.
Ethyl methylphenylglycidate’s flavor and aroma are reminiscent of strawberries, hence its nickname. It is used not only as an artificial strawberry-flavoring agent, but also in the fragrance industry, particularly in perfumes, personal care products, soaps, and detergents.
So why might you be consuming this molecule on Saturday? January 15 is National Strawberry Ice Cream Day! Be sure to get your fill of ethyl methylphenylglycidate.
Ethyl methylphenylglycidate hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Skin sensitization, category 1B | H317—May cause an allergic skin reaction | |
| Serious eye damage/irritation, category 2A | H319—Causes serious eye irritation | |
| Short-term (acute) aquatic hazard, category 2 | H401— Toxic to aquatic life | |
| Long-term (chronic) aquatic hazard, category 2 | H411—Toxic to aquatic life with long-lasting effects | |
*Compilation of two safety data sheets.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
MOTW update
Dimethyl sulfoxide (DMSO) was the Molecule of the Week for September 20, 2021. It is a valuable laboratory and industrial solvent that has been used and abused as a medical treatment. A 2016 report showed that it may offer relief for autoimmune arthritis patients.
Just recently, another use for DMSO was discovered. Jie-Ping Wan and colleagues at Jiangxi Normal University (Nanchang, China) reported that DMSO is a useful C1 source in organic synthesis. They described an iodine-catalyzed [2 + 2 +1] cascade reaction among enaminones, hydrazines, and DMSO that produces pyrazole derivatives which may prove valuable for making new drugs. DMSO is also the solvent for the reaction.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Ethyl methylphenylglycidate
fast facts
| CAS Reg. No. | 77-83-8 |
| SciFinder nomenclature | 2-Oxiranecarboxylic acid, 3-methyl-3-phenyl-, ethyl ester |
| Empirical formula | C12H14O3 |
| Molar mass | 206.24 g/mol |
| Appearance | Colorless to pale yellow liquid |
| Boiling point | 272–275 °C |
| Water solubility | <1 g/L |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

