What molecule am I?
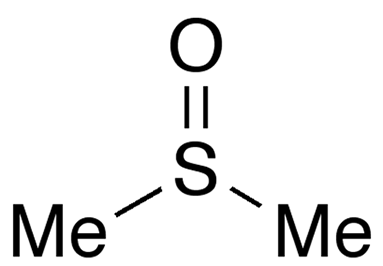
Dimethyl sulfoxide (DMSO) is a widely used solvent that is miscible with water and a wide range of organic solvents. It goes by several names, including methyl sulfoxide, sulfinylbismethane, and dozens of trade names.
DMSO was first discovered in the late 19th century as a byproduct of the kraft process for making paper from wood pulp. About the same time, Russian chemist Alexander Zaytsev synthesized it by oxidizing dimethyl sulfide, another kraft process byproduct. Zaytsev’s synthesis is the basis for the manufacturing process still used today.
DMSO is a laboratory and industrial solvent for many gases, synthetic fibers, paint, hydrocarbons, salts, and natural products. Because it is aprotic, relatively inert, nontoxic, and stable at high temperatures, it is a frequently used solvent for chemical reactions. Its deuterated form is an ideal solvent for NMR spectroscopy.
In the 1960s, scientists observed that DMSO penetrates human skin with little effect on tissues; and the solvent was tested as a way for medicines to be carried into the body as an alternative to oral formulations or injectables. Since then, DMSO has been used in some transdermal drug delivery systems (i.e., patches). In 1978 the US Food and Drug Administration approved it for use for the symptomatic relief of chronic interstitial cystitis (bladder pain syndrome)—the only FDA approval for DMSO as an actual medication.
As one might expect for the 1960s, DMSO was tried as an alternative drug for inflammation relief and as a solvent for introducing illicit drugs such as cocaine into the body. It was also wrongly touted as a cancer cure. In 1965, FDA put the kibosh on much of this activity by banning clinical trials with DMSO because the compound altered the refractive index of eye lenses of laboratory animals. The ban was lifted in 1980 after the intense interest in the substance abated.
Researchers continue to look at DMSO as a possible medical treatment. In 2016, Gerald Krystal and colleagues at the British Columbia Cancer Agency (Vancouver), the University of British Columbia (Vancouver), and Vancouver General Hospital reported that DMSO represses inflammatory cytokine production from human blood cells and thus reduces autoimmune arthritis. The authors also examined whether DMSO has any anticancer activity; they concluded that they could not confirm that it does.
Dimethyl sulfoxide hazard information
| Hazard class* | GHS code and hazard statement |
|---|---|
| Flammable liquids, category 4 | H227 —Combustible liquid |
*Globally Harmonized System (GHS) of Classification and Labeling of Chemicals.
Molecule of the future
The CRISPR-Cas9 genome-editing system, which won the 2020 Nobel Prize in Chemistry for Emmanuelle Charpentier and Jennifer Doudna, revolutionized biology; but it is not as efficient as it might be. It inserts a new DNA fragment at the targeted location in a cell’s existing DNA less than 20% of the time, often because the cell repairs the break before the insertion can be completed.
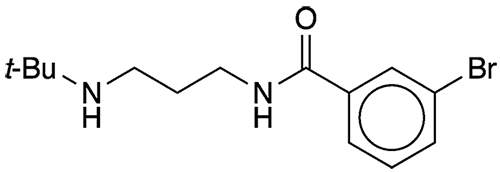
As reported at last month’s ACS national meeting, Lindsey James* and Caroline Foley at the University of North Carolina at Chapel Hill are seeking molecules that block the action of a protein that facilitates rapid DNA repair. The researchers began with the bromobenzamide derivative UNC21701 (shown); it showed some promise, but its low affinity for the protein, p53 binding protein 1 (53BP1), made it only a partial solution.
James and Foley screened 1200 additional candidates, including a promising acylpiperidine derivative. In their meeting abstract, the authors conclude, “Based on our preliminary data, we believe that the development of a high-quality chemical probe of 53BP1 would provide a universal reagent to enable more efficient gene editing using CRISPR-Cas9, thereby benefitting basic researchers and gene-directed therapeutics as well.”
1. CAS Reg. no. 1648707-58-7.
Molecule of the Future
Once a month we bring you a newly discovered or developed molecule that has important implications for the future of chemistry or society in general. Look for it the third week of each month. Learn more about this month's Molecule of the Future.
We're looking for more molecules of the future!
Do you have a suggestion for the next molecule of the future? Send your idea to MOTW.
Dimethyl sulfoxide
fast facts
| CAS Reg. No. | 67-68-5 |
| SciFinder nomenclature | Methane, 1,1’-sulfinylbis- |
| Empirical formula | C2H6OS |
| Molar mass | 78.13 g/mol |
| Appearance | Colorless liquid |
| Melting point | 19°C |
| Boiling point | 189 °C |
| Water solubility | Miscible |
MOTW update:
January 10, 2022
Dimethyl sulfoxide (DMSO) is a valuable laboratory and industrial solvent that has been used and abused as a medical treatment. A 2016 report showed that it may offer relief for autoimmune arthritis patients.
Just recently, another use for DMSO was discovered. Jie-Ping Wan and colleagues at Jiangxi Normal University (Nanchang, China) reported that DMSO is a useful C1 source in organic synthesis. They described an iodine-catalyzed [2 + 2 +1] cascade reaction among enaminones, hydrazines, and DMSO that produces pyrazole derivatives which may prove valuable for making new drugs. DMSO is also the solvent for the reaction.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.