What molecule am I?
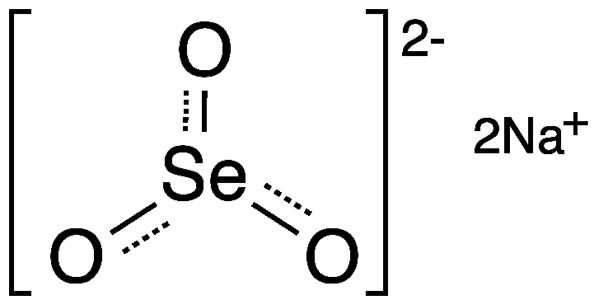
Sodium selenite (Na2SeO3) is a toxic inorganic salt that is highly soluble in water and insoluble in organic solvents. The crystal structure of the anhydrous salt has been variously reported as tetragonal or monoclinic.
In 1889, Irish chemists Sir Charles A. Cameron* and John Macallan produced Na2SeO3 and the previously unknown selenium oxychloride1 (SeOCl2) by heating selenium oxide (SeO2) with sodium chloride. This finding was reported in a pioneering series of articles on selenium chemistry.
In an improved 1929 synthesis, J. B. Krak at the Roessler and Hasslacher Chemical Co. (Perth Amboy, NJ) prepared Na2SeO3 by evaporating a solution of sodium hydroxide and selenious acid (H2SeO3) at 60–100 °C. Commercial Na2SeO3 is now made by heating an aqueous solution of SeO2 and NaOH to form its pentahydrate2, which is crystallized and then warmed to 40 °C to liberate the anhydrous salt.
Na2SeO3 and other selenite salts are used to “neutralize” green impurities during the manufacture of colorless glass. Iron-containing impurities in the natural silicas (e.g., sand) used to make glass absorb red and blue wavelengths of light, giving it a green appearance. Selenites have no color; but when they are dissolved in the glass mixture, they appear pinkish, counteracting the green hue and making the glass colorless.
Sodium selenite hazard information
| Hazard class* | GHS code and hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 2 | H300—Fatal if swallowed | |
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Skin sensitization, category 1 | H317—May cause an allergic skin reaction | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Acute toxicity, inhalation, category 2 | H330—Fatal if inhaled | |
| Short-term (acute) aquatic hazard, category 2 | H401—Toxic to aquatic life | |
| Long-term (chronic) aquatic hazard, category 2 | H411—Toxic to aquatic life with long-lasting effects | |
*Globally Harmonized System (GHS) of Classification and Labeling of Chemicals.
Explanation of pictograms.
MOTW update
Tetrodotoxin was the Molecule of the Week for April 5, 2010. It is an extremely toxic natural substance—the poisonous principle in pufferfish and other marine animals. Tetrodotoxin, a nerve blocker, has been tested as a local anesthetic, but its concentration range must be limited to prevent it from becoming systemically toxic. It is not used currently for clinical purposes.
Anesthesiologist Daniel S. Kohane and his team at Boston Children’s Hospital and Harvard Medical School developed a self-assembling drug delivery system that releases the nerve-blocker more slowly and with less toxicity than direct injection. In experiments with rats, they used modified versions of peptides from the same voltage-gated sodium channels that tetrodotoxin targets to deliver the drug. The slower release allowed the toxin to block the rats’ sciatic nerves >3 times as long as free tetrodotoxin, without harmful effects.
See this week's MOTW update below.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Sodium selenite fast facts
| CAS Reg. No. | 10102-18-8 |
| SciFinder nomenclature | Selenious acid, sodium salt (1:2) |
| Empirical formula | Na2O3Se |
| Molar mass | 172.94 g/mol |
| Appearance | Colorless crystals or white powder |
| Melting point | 720° C (dec.)a |
| Water solubility | 900 g/L |
a. Decomposition temperatures as low as
320 °C are reported.
Over the years, readers have noted that ionic substances are not actually molecules. This is correct, but we use "molecules" in the broadest sense to include them in Molecule of the Week.—Ed.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

