What molecule am I?
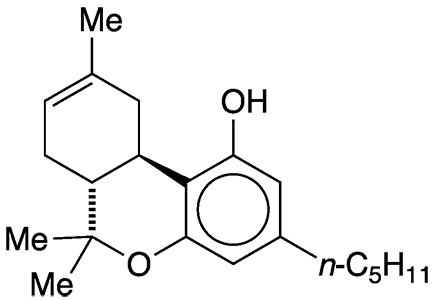
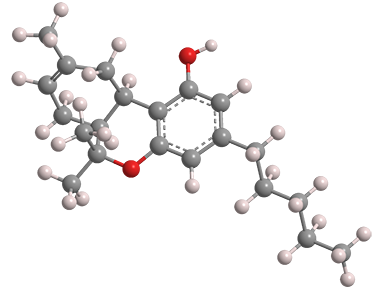
Δ8-Tetrahydrocannabinol (Δ8-THC) is a positional isomer of the more abundant and widely known Δ9-tetrahydrocannabinol1 (Δ9-THC), which is usually called just THC. The Δ9 isomer was the Molecule of the Week for January 24, 2011.
In contrast to Δ9-THC, the Δ8 isomer content of marijuana plants (Cannabis spp.) is no more than 1%. It is most likely not produced by plant metabolism but is formed by the degradation (isomerization) of Δ9. The pharmacological profile of Δ8 is similar to that of Δ9 with somewhat less psychoactive potency.
Richard L. Hively and William A. Mosher at the University of Delaware (Newark) and Friedrich W. Hoffmann at Edgewood Arsenal (MD) reported the isolation of Δ8-THC in 1966, just 2 years after the initial report of the structure of Δ9.2 One year later, the research team that pioneered the discovery of THC, led by Raphael Mechoulam at the Hebrew University of Jerusalem, published stereospecific total syntheses of both isomers.
Since its discovery and synthesis, Δ8-THC has been largely ignored by the public—until recently. Even though the psychoactive properties of Δ8 are like those of the Δ9 isomer, it is largely unregulated; and consumers have begun to use it to achieve a high while relieving stress and anxiety. The low concentration of Δ8 in cannabis is overcome by synthesizing it from widely available, inexpensive cannabidiol; however, the resulting product is usually impure, and its health effects have not been extensively studied.
As the Δ8-THC craze spreads, chemists and regulators are growing increasingly concerned. Several states have begun to crack down on sales even though Δ8-THC that contains <0.3% of the Δ9 isomer is not illegal. The president of one drug-testing firm says, “I believe that Δ8 has a legitimate place in therapeutics and potentially adult use, but I just don’t see anybody doing it appropriately. It’s all bathtub gin.”
1. CAS Reg. No. 1972-08-3.
2. In the nomenclature system used in this and other early articles about THCs, Δ9 is named Δ1, and Δ8 is named Δ6.
Δ8-Tetrahydrocannabinol hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 4 | H302—Harmful if swallowed | |
*Information based on safety data sheets for Δ9-tetrahydrocannabinol.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
MOTW update: October 11, 2021
Δ8-Tetrahydrocannabinol is a minor constituent of marijuana that has come into increased use in recent years. In response to a C&EN report on Δ8-THC, readers reacted with additional information and views concerning this molecule.
Δ8-Tetrahydrocannabinol
fast facts
| CAS Reg. No. | 5957-75-5 |
| SciFinder nomenclature | 6H-Dibenzo[b,d]pyran-1-ol, 6a,7,10,10a-tetrahydro-6,6,9-trimethyl-3-pentyl-, (6aR,10aR)- |
| Empirical formula | C21H30O2 |
| Molar mass | 314.46 g/mol |
| Appearance | Colorless oil |
| Boiling point | ≈200° C (0.02 torr)a |
| Water solubility | 3 mg/La |
a. Estimates based on data for Δ9-tetrahydrocannabinol.
MOTW update
Dihydrolevoglucosenone was the Molecule of the Week for March 5, 2019. It is a biobased, biodegradable solvent that has the potential to replace toxic aprotic solvents such as N,N-dimethylformamide and N-methyl-2-pyrrolidone. The product’s developer, Circa Group (Parkville, Australia), recently announced a site in northeastern France for a commercial plant that will make 1000 t/year of dihydrolevoglucosenone from waste cellulose.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

