What molecule am I?
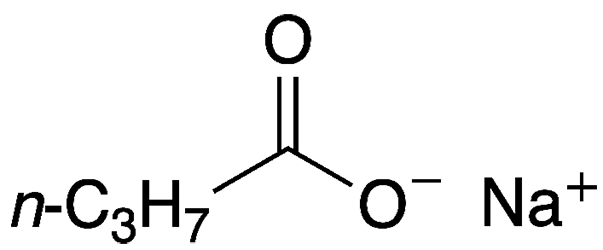
Sodium butyrate—more properly sodium butanoate—is a salt of butyric (butanoic) acid. If it comes into contact with even a trace of moisture, it emits a faint odor of its foul-smelling conjugate acid.
The butyrate ion, along with the analogous propionate ion, is purported to enhance gut health in humans and many other animal species. But you don’t have to take it as a supplement. Bacteria in the gut produce butyrate from dietary fiber, especially the fiber from legumes and nuts.
Two recent reports illustrate the benefits of butyrate. Yanfen Bai and Thomas J. Mansell* at Iowa State University (Ames) wrote, “The short-chain fatty acid [anion] butyrate plays critical roles in human gut health, affecting immunomodulation, cell differentiation, and apoptosis, while also serving as the preferred carbon source for colon cells.” To assist in studies of butyrate for therapeutic applications, the researchers developed a high-throughput biosensor that responds to intracellular concentrations.
In another account, Sanne Verhoog at the University of Bern (Switzerland) and collaborators there and at other institutions in Switzerland, Turkey, Germany, and the United States reviewed 29 studies about how dietary factors influence the populations of two beneficial gut bacteria, Akkermansia muciniphila (11 studies) and Faecalibacterium prausnitzii (25 studies). The studies involved a total of 1444 participants. Supplementation with several substances, including sodium butyrate, increased the abundance of A. muciniphila, whereas diets low in fermentable saccharides that produce butyrate lowered the quantity of the bacterium. (Butyrate was not considered in the case of F. prausnitzii.)
Sodium butyrate hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 4 | H302—Harmful if swallowed | |
| Skin corrosion/irritation, category 1B | H314—Causes severe skin burns and eye damage | |
| Serious eye damage/eye irritation, category 1 | H318—Causes serious eye damage | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
| Germ cell mutagenicity, category 2 | H341—Suspected of causing genetic defects | |
| Short-term (acute) aquatic hazard, category 3 | H402—Harmful to aquatic life | |
*Compilation of selected safety data sheets.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
MOTW updates
Δ8-Tetrahydrocannabinol was the Molecule of the Week for October 4, 2021. It is a minor constituent of marijuana that has come into increased use in recent years. In response to a C&EN report on Δ8-THC, readers reacted with additional information and views concerning this molecule.
Molybdenum disulfide (MoS2) was the Molecule of the Week for February 24, 2020. It is an excellent “dry” lubricant, a semiconductor, and an important molecule in superconductivity research. Now, Jiwoong Park at the University of Chicago, David Cahill at the University of Illinois at Urbana–Champaign, and Paul Erhart at the Chalmers University of Technology (Gothenburg, Sweden) report that stacked sheets of MoS2 form a strong thermal conductor in two dimensions but block heat transfer between layers. The 900-fold heat-conduction ratio breaks the record set by single-crystal graphite by a factor of 3.
See this week's MOTW updates below.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Sodium butyrate fast facts
| CAS Reg. No. | 156-54-7 |
| SciFinder nomenclature | Butanoic acid, sodium salt (1:1) |
| Empirical formula | C4H7NaO2 |
| Molar mass | 110.09 g/mol |
| Appearance | White crystals or powder |
| Melting point | 250–253° C |
| Water solubility | 0.5–100 g/La |
a. A wide range of solubilities are reported in the literature. Readers are invited to submit the generally accepted value to motw@acs.org.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

