What molecule am I?
L-Lysine is one of the nine essential amino acids—essential because the human body cannot synthesize them so they must therefore be included in a healthy diet. It is one of the four common α-amino acids to have a nitrogen atom in its side chain.
In 1889, Edmund Drechsel at the University of Freiburg (Germany) isolated lysine by hydrolyzing casein, a protein found in milk. The molecule’s structure was elucidated in 1902 by Emil Fischer and Fritz Weigert at the University of Berlin; they synthesized it and compared it with the natural product.
Fortunately for humans, lysine is abundant in a wide range of high-protein foods, including red meat, some fish, eggs, cheese, and legumes. As with most amino acids, lysine’s key role in the body is the formation of proteins.
Why are we featuring lysine during National Chemistry Week? The theme for this year’s NCW is catalysis—and lysine plays a key role in a biocatalytic–organocatalytic process reported last year by Kelsey N. Stewart, Emily G. Hicks, and Dylan W. Domaille* at the Colorado School of Mines (Golden).
The researchers sought to biosynthesize value-added chemicals from renewable feedstocks by using unmodified (i.e., natural) microorganisms rather than engineered ones. They developed a process in which they combined the bacterium Gluconobacter oxidans1 and lysine as cocatalysts for the single-pot conversion of straight-chain aliphatic alcohols to α,β-unsaturated aldehydes. The reaction takes place in water under mild conditions; it is not necessary to isolate the intermediate products.
The specific role of lysine is to prevent the reaction from diverting to a simple oxidation of the starting alcohol to a carboxylic acid. The authors’ proof-of-concept reaction is the conversion of 1-butanol to 2-ethyl-2-hexenal, a chemical that is currently manufactured on a multimillion t/year scale from nonrenewable fossil-fuel feedstocks with rhodium catalysis at elevated temperature and pressure.
The authors conclude, “our study highlights that merging chemical catalysis with in situ biocatalysis is a powerful strategy to increase the complexity and breadth of products available from bioprocesses.”
1. Also known as Gluconobacter oxydans; found in spoiled (oxidized) fruit and fermented beverages.
L-Lysine hazard information
| Hazard class* | GHS code and hazard statement |
|---|---|
| Not a hazardous substance or mixture |
Molecule of the future
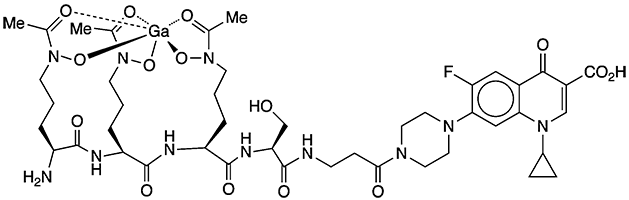
Ciprofloxacin, the Molecule of the Week for May 30, 2016, is a widely used, inexpensive, broad-spectrum antibiotic. But there’s always room for improvement. At the ACS fall 2021 national meeting, Eszter Boros and colleagues at Stony Brook University (NY) described a souped-up version of the drug, called galbofloxacin, that consists of a gallium(III)-based siderophore attached to the ciprofloxacin molecule via a serine linkage (see figure).
Harmful bacteria use siderophores to scavenge iron from their hosts. Adding the Ga(III) siderophore to ciprofloxacin causes the bacteria to imbibe gallium instead of iron, fouling up their cellular processes and ultimately killing them. The one–two punch of gallium and ciprofloxacin allows the use of lower doses of the antibiotic to control infections.
Molecule of the Future
Once a month we bring you a newly discovered or developed molecule that has important implications for the future of chemistry or society in general. Look for it the third week of each month. Learn more about this month's Molecule of the Future below.
We're looking for more molecules of the future!
Do you have a suggestion for the next molecule of the future? Send your idea to MOTW.
L-Lysine fast facts
| CAS Reg. No. | 56-87-1 |
| SciFinder nomenclature | L-Lysine |
| Empirical formula | C6H14N2O2 |
| Molar mass | 146.19 g/mol |
| Appearance | White crystals or powder |
| Melting point | 224.5 °C (dec.) |
| Water solubility | >1 kg/L |
MOTW update:
September 5, 2022
L-Lysine is an essential amino acid that is also valuable as a cocatalyst in a bacterium-driven aldehyde synthesis.
Now lysine has taken on a new role in cancer drug development. For attacking cancer cell proteins, covalent bonding of a drug to protein amino acids is preferable to other forms of attachment because covalent bonding is irreversible and allows the drug to remain longer at the attachment site. Until now, cysteine has been the only amino acid targeted covalently, but it has limited utility because it is relatively rare in cancer proteins.
Recently, scientists at Terremoto Biosciences (San Francisco) began to study lysine as a target. Lysine is attractive because, after cysteine, it is the most nucleophilic amino acid and therefore highly reactive. Terremoto’s work is in the early stages; a practical drug is likely several years off.
1. CAS Reg. No. 202650-88-2.
2. CAS Reg. No. 872993-05-0.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.