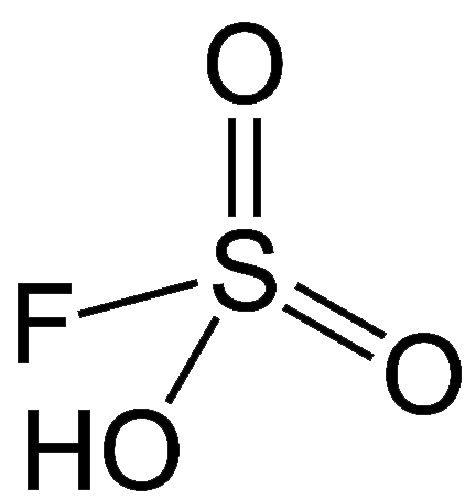
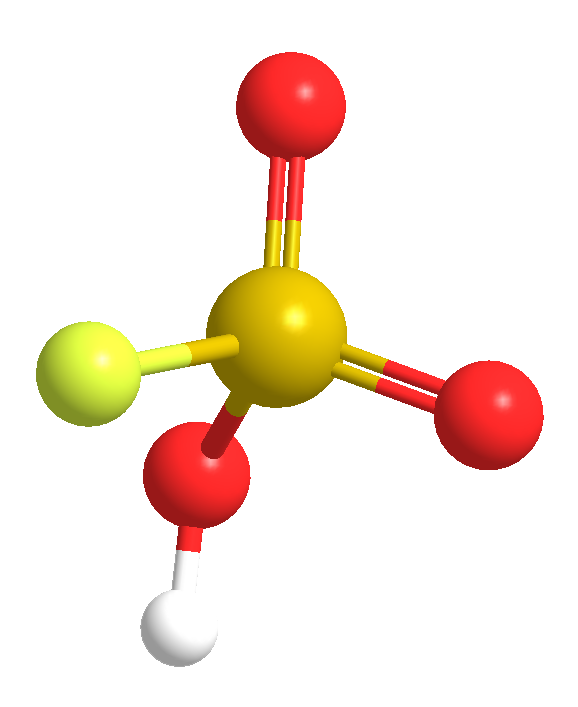

This week’s molecules combine to make “magic”. The first, fluorosulfuric acid (HSO3F; also called fluorosulfonic acid) is an extremely strong Brønsted acid that has been known since the late 19th century. It is ≈1000 times stronger than pure sulfuric acid, which puts it in the class of superacids. It can protonate and dissolve almost all organic compounds and has several industrial uses, including catalysis and hydrofluorination.
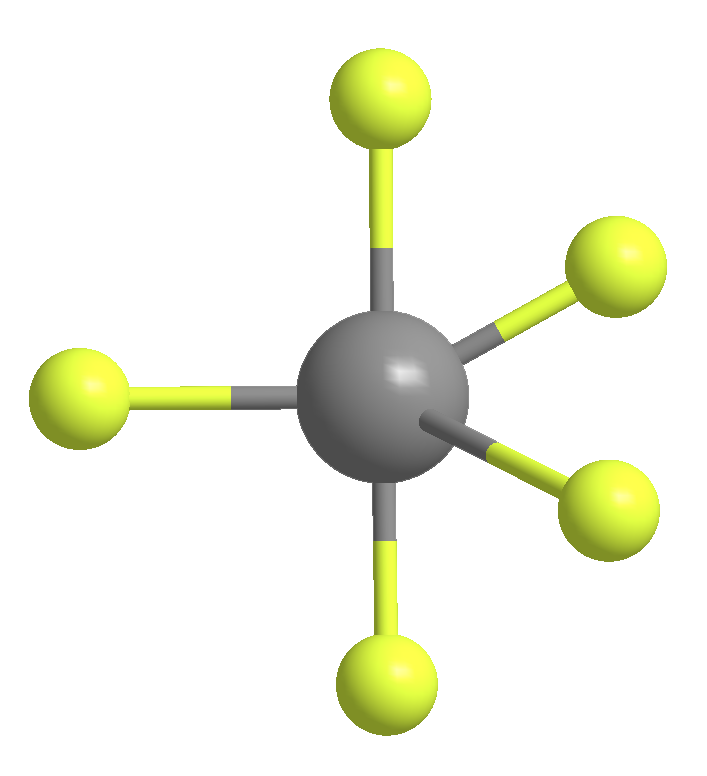
The second molecule, antimony pentafluoride (SbF5), was first reported in 1904. This strong Lewis acid is nasty: It reacts violently with water, attacks the skin and eyes, and corrodes metals. Its chief use is the fluorination of organic compounds.
So where’s the magic? In the 1960s, George A. Olah and co-workers, first at Dow Chemical (Midland, MI), then at Case Western Reserve University (Cleveland), were seeking ways to make and study stable carbocations. To extract hydride groups (hydrogen anions) from organic compounds, they needed a strong, but poorly nucleophilic, acid.
At Case Western, the researchers discovered that a mixture of HSO3F and SbF5easily dissolves paraffins. NMR spectra of the solutions showed that the acidic combination cleaved the long-chain alkanes, and the fragments isomerized to form stable tert-butyl cations (Me3C+). This result was so spectacular that the chemists dubbed the mixture “magic acid”. The usual molar ratio of the components is 1:1, but other combinations have also been useful.
Olah went on to do extensive research on carbocations at Case Western and then the University of Southern California (Los Angeles). He received the Nobel Prize in Chemistry in 1994 for his pioneering work.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

