August 3 is National Watermelon Day, and so this week’s molecules have a watermelon flavor.
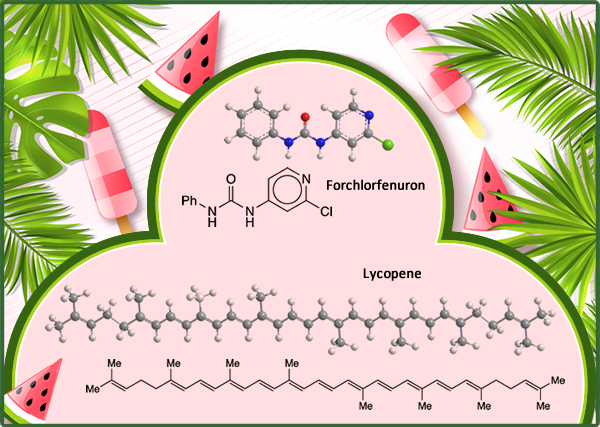
Lycopene is the source of the red color in several fruits, notably watermelon (Citrullus lanatus var. lanatus) and tomato (Solanum lycopersicum). The pigment, also known as ψ,ψ-carotene, is a linear polyolefin that contains eight isoprene units. It has 13 double bonds, all in the E-configuration.
In 1910, Swiss chemists Richard M. Willstätter and Heinrich H. Escher isolated lycopene from tomatoes and determined its structure. But not until 1950 did another Swiss chemist, Paul Karrer, and his co-workers synthesize it.
Lycopene is an antioxidant that may have some health benefits, particularly against cancer and cardiovascular diseases. But agencies such as the European Food Safety Authority and the US Food and Drug Administration are not convinced; on the basis of current evidence, they have refused to label lycopene as a beneficial drug.
Forchlorfenuron, formally 1-(2-chloropyridin-4-yl)-3-phenylurea (CPPU), is a growth regulator that increases the size (and therefore the value) of such fruits as apple, cherry, and kiwi. It’s been used on watermelon too; but at least once, the results were unexpected.
In China in 2011, about 20 farmers applied too much CPPU on their watermelon crops too late in the growing season. The weather conditions were ideal, so much so that the watermelons grew too fast and exploded. The watermelon fragments, about 46 hectares worth, were used to feed fish and pigs.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

