What molecule am I?
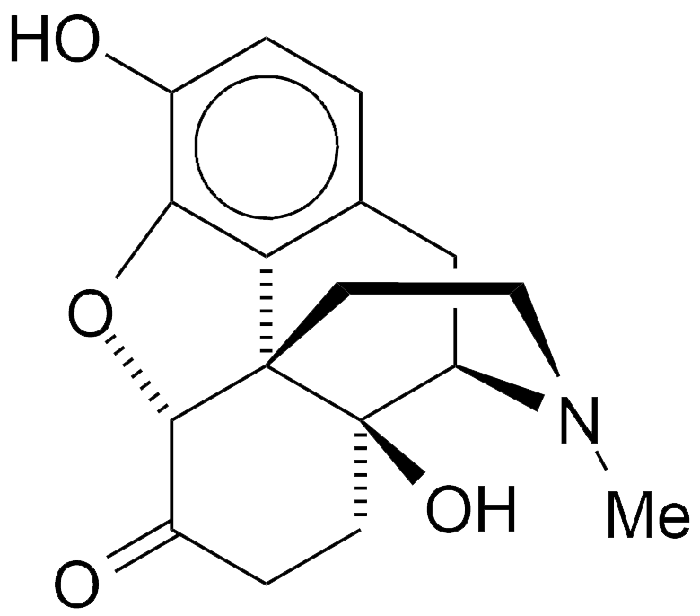
Oxymorphone is a 60-year-old opioid analgesic. Its structure is that of morphine with the double bond hydrogenated and an additional hydroxyl group that makes it 10 times more potent than morphine.
In 1955, Ulrich Weiss at the New York Botanical Garden (of all places) began a series of articles on new morphine derivatives. In the first article of the series, he described the synthesis of 14-hydroxydihydromorphinone, which later became known as oxymorphone. Weiss went on to publish five more articles on morphine derivatives.
In 1957, a patent on the synthesis was awarded to Weiss and Mozes Juda Lewenstein. (The patent application preceded Weiss’s article.) The patent was assigned to Lewenstein of Endo Pharmaceuticals (New York). Endo began to market the drug in the United States in 1959.
In June of this year, the US Food and Drug Administration, concerned about the widespread abuse of opioids, requested that the manufacturer (now Endo International, based in Dublin, Ireland) withdraw oxymorphone-containing Opana ER from the market. Although Endo formulated the solid pill Opana ER to prevent users from snorting or injecting it, people still figured out ways to abuse the drug.
In July, Endo “voluntarily” removed Opana ER while stressing that the drug is safe and efficacious when used properly.
Oxymorphone fast facts
| CAS Reg. No. | 76-41-5 |
| Molar mass | 301.34 g/mol |
| Formula | C17H19NO4 |
| Appearance | White crystals |
| Melting point | 248–249 °C (dec.) |
| Water solubility | 24 g/L |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

