What molecule am I?
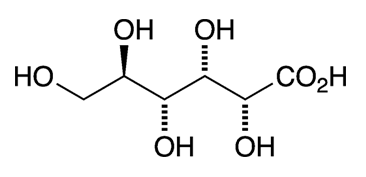
D-Gluconic acid is the oxidized form of D-glucose (or dextrose), one of the fundamental building blocks for sugars, polysaccharides, and cellulose. Like glucose, it cyclizes in solution, in this case to form an ester (glucono-δ-lactone) rather than a hemiacetal.
Gluconic acid widely exists in nature, especially in fruits and in sucrose-containing substances such as honey. Early methods of synthesizing gluconic acid from glucose included hypobromite oxidation and alkaline hydrolysis. Now it is commercially produced by using microbes such as Aspergillus niger to oxidize glucose enzymatically.
Gluconate, gluconic acid’s conjugate base, is useful as a metal-chelating agent in alkaline solutions. It is a component of many cleaning products; and it is used to prevent formation of solids in dairy processing and beer brewing.
More recently, gluconic acid has been investigated as a chelating agent for extracting rare earths (lanthanides) from the fertilizer waste phosphogypsum.1 An estimated 100,000 t per year of valuable lanthanides are discarded in this waste product worldwide. Sulfuric acid is highly effective for recovering the rare earths, but a team led by Richard E. Riman of Rutgers University (New Brunswick, NJ) showed that gluconic acid and other bioacids are promising alternatives and would be considerably easier than sulfuric acid to treat for disposal.
1. Phosphogypsum does not contain phosphorus; it is a mostly gypsum (CaSO4·2H2O) waste product of phosphoric acid plants.
D-Gluconic acid hazard information
| GHS classification*: skin corrosion/irritation, category 2 | |
| H315—Causes skin irritation | |
| GHS classification: serious eye damage/eye irritation, category 2A | |
| H319—Causes serious eye irritation | |
*Globally Harmonized System of Classification and Labeling of Chemicals. Explanation of pictograms.
D-Gluconic acid fast facts
| CAS Reg. No. | 526-95-4 |
| Empirical formula | C6H12O7 |
| Molar mass | 196.16 g/mol |
| Appearance | Colorless or white crystals |
| Melting point | 131 ºC |
| Water solubility | 316 g/L |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

