What molecule am I?
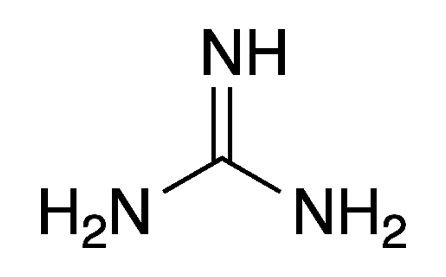
Guanidine is a small, nitrogen-rich organic compound found in nature in plants (e.g., rice hulls and turnip juice) and animals (e.g., mussels and earthworms). Unlike its oxygen analogue, carbonic acid, it exists at ambient conditions (i.e., not in solution nor at cryogenic temperatures). Guanidine should not be confused with guanine, a purine derivative that is found in bat and bird feces.
Many literature sources state that guanine is very soluble in water; but, as shown in the fast facts table, it is only slightly soluble. It is, however, very hygroscopic. Its hydrochloride salt is highly water-soluble and is the usual article of commerce. The free base is extremely toxic, as shown in the hazard information table.
Guanidine was discovered in nature in the late 19th century. In 1907, a German patent was awarded to Italian chemist Celso Ulpiani for the reaction of dicyanamide with strong acid to produce guanidine (actually, guanidinium) nitrate. Twenty-four years later, G.B.L. Smith, V. J. Sabetta, and O. F. Steinbach, Jr., at the Polytechnic Institute of Brooklyn (now New York University Tandon School of Engineering) published a comprehensive study of the conversion of dicyandiamide (aka 2-cyanoguanidine) to guanidinium nitrate and then to nitroguanidine, a powerful explosive.
Guanidine’s hydrothiocyanate salt has an interesting connection to the current COVID-19 pandemic. This past June, the US Food and Drug Administration warned laboratories not to use media that contain the thiocyanate salt or other guanidine-based chemicals to transport COVID-19 samples. The reason: Guanidine compounds can decompose to highly toxic hydrogen cyanide (HCN) gas. The FDA also advised that when personnel do not know the ingredients of a transport medium, they should handle it as though it contained a guanidine product. As of the date of FDA’s warning, no HCN-related injuries had been reported to the agency.
Guanidine hazard information
| Hazard class* | Hazard statement | |
|---|---|---|
| Flammable liquids**, category 2 | H225—Highly flammable liquid and vapor | |
| Acute toxicity, oral, category 3 | H301—Toxic if swallowed | |
| Acute toxicity, dermal, category 3 | H311—Toxic in contact with skin | |
| Skin corrosion/irritation, category 1 | H314—Causes severe skin burns | |
| Serious eye damage/eye irritation, category 1 | H318—Causes serious eye damage | |
| Acute toxicity, inhalation, category 3 | H331—Toxic if inhaled | |
| Specific target organ toxicity, single exposure, category 1 | H370—Causes damage to organs | |
*Globally Harmonized System of Classification and Labeling of Chemicals.
Explanation of pictograms.
**The low melting point of guanidine qualifies it as a hazardous liquid.
Guanidine fast facts
| CAS Reg. No. | 113-00-8 |
| SciFinder nomenclature | Guanidine |
| Empirical formula | CH5N3 |
| Molar mass | 59.07 g/mol |
| Appearance | Hygroscopic white crystals |
| Melting point | 50 ºC |
| Water solubility | 1.84 g/L |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

