What molecule am I?
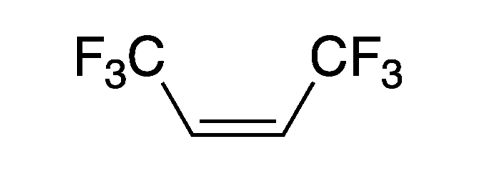
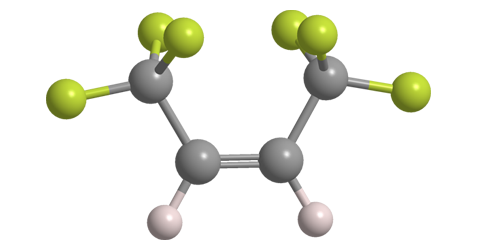
Hexafluoro-2-butene, more specifically (Z)-1,1,1,4,4,4-hexafluoro-2-butene, is an unsaturated, highly fluorinated hydrocarbon with multiple industrial uses. It also goes by the rather bizarre commercial name 1336mzz(Z).
Hexafluoro-2-butene is used as a working fluid for organic Rankine power cycles, as a refrigerant for use in chillers, and as a foam-blowing agent. (A Rankine cycle is a construct used to evaluate the performance of steam turbine systems; “organic” refers to the use of an organic liquid with a lower boiling point than water in such a system.)
Despite the harmful properties shown in the hazard information table, the U.S. Environmental Protection Agency approved its industrial uses in 2017 under the agency’s Significant New Alternatives Policy. The driving force for this approval was hexafluoro-2-butene’s short atmospheric lifetime of 22 days that contrasts with ≈8 years for hydrofluorocarbon HFC-245fa, which has similar uses. The compound’s labile carbon–carbon double bond is the key to its short lifetime.
Another advantage of hexafluoro-2-butene is its very low 100-year global warming potential of 2 (carbon dioxide = 1) compared with 858 for HFC-245fa. It is relatively nontoxic and not flammable.
Although hexafluoro-2-butene was synthesized as early as 1952 by Robert N. Haszeldine at the University of Cambridge (UK), few of its thermodynamic and other physical properties have been published. Knowledge of hexafluoro-2-butene’s properties is important for manufacturers and users of industrial equipment, so Mark O. McLinden* at the National Institute of Standards and Technology (Boulder, CO) and Ryo Akasaka at Kyushu University (Fukuoka, Japan) undertook a comprehensive study of its properties.
The authors measured experimental data for the vapor pressure (including dew points), density, and vapor-phase sound speeds for hexafluoro-2-butene. Using these and previously reported data, they developed an equation of state based on Helmholtz free energy for the compound. The equation is valid for the temperature range 200–500 K and pressures ≤46 MPa.
(Z)-1,1,1,4,4,4-Hexafluoro-2-butene hazard information
| Hazard class* | Hazard statement | |
|---|---|---|
| Gases under pressure, liquefied gas | H280—Contains gas under pressure; may explode if heated | |
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
| Specific target organ toxicity,single exposure, narcotic effects, category 3 | H336—May cause drowsiness or dizziness | |
| Simple asphyxiant, category 1 | H380—May displace oxygen and cause rapid suffocation | |
*Globally Harmonized System of Classification and Labeling of Chemicals.
Explanation of pictograms.
(Z)-1,1,1,4,4,4-Hexafluoro-2-butene fast facts
| CAS Reg. No. | 692-49-9 |
| SciFinder nomenclature | 2-Butene, 1,1,1,4,4,4-hexafluoro-, (2Z)- |
| Empirical formula | C4H2F6 |
| Molar mass | 164.05 g/mol |
| Appearance | Colorless liquid |
| Boiling point | 33.0–33.5 ºCa |
| Water solubility | 0.76 g/L |
a. Some sources give the boiling point as 8.5–9.0 ºC, but this is the value for the (E) isomer.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

