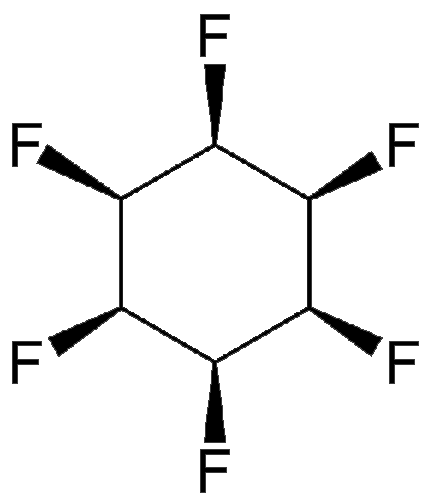
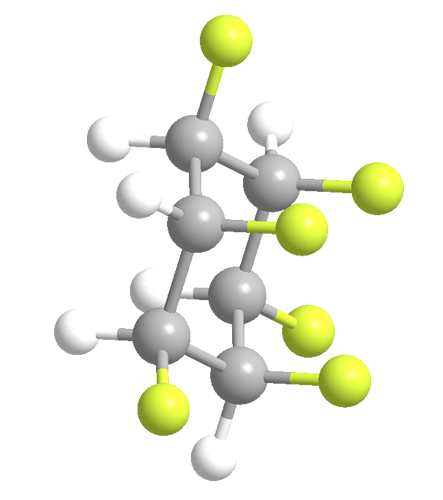
What’s the most polar nonionic compound? In March 2015 at the ACS national meeting in Denver, D. O’Hagan and co-workers at the University of St. Andrews (UK) described a molecule that they claim holds this title. In 12 steps, they synthesized all-cis-hexafluorocyclohexane (HFC) from inositol, a remarkable feat because of the specific required stereochemistry and the repulsive forces among the six fluorine atoms on the same side of the ring.
Because the molecule is electropositive on one side of the ring and strongly electronegative on the other, it has the large dipole moment of 6.2 D (debyes). (A compound’s polarity is measured by its dipole moment.) The O’Hagan team could not find a report of a more polar nonionic compound in the literature.
Soon after O’Hagan’s report, J. Meany of Tuscaloosa, AL, said, “Not so fast.” He pointed out that nonionic donor–acceptor pairs of organic molecules have much higher (15–20 D) dipole moments. He suggests that all-cis-HFC be called the “most polar aliphatic molecule”.
The following month, A. F. Pozharskii of Rostov-on-Don, Russia, chimed in. In 1991, he and his co-workers reported the synthesis of 4,5-bis(dimethylamino)-1,8-naphthalenedicarboxaldehyde* with a dipole moment of 9.2 D. He echoes Meany by stating that “all-cis HFC is probably the most polar among now-known fully saturated nonionic compounds.”
* Zh. Org. Khim. [Russian Journal of Organic Chemistry] 1991, 27, 1536.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

