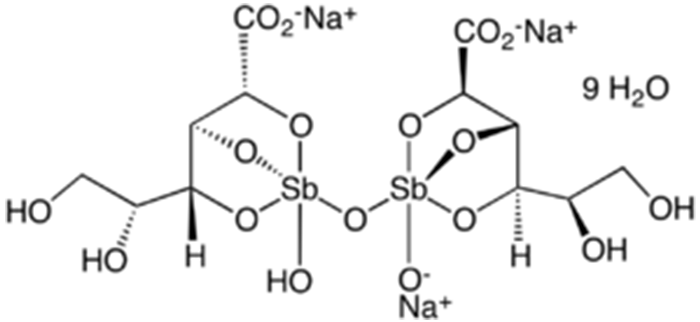
Sodium stibogluconate (Na3Sb2C12H38O26), also known as sodium antimony(V) gluconate, is a solid ionic compound that is freely soluble in water. Its precise structure is unknown; the one shown here is idealized based on the atomic formula.
Workers in India prepared sodium stibogluconate in the late 1940s via the reaction of antimony pentoxide with sodium hydroxide. It has been used since then as an injectable drug for treating visceral leishmaniasis, a fatal insect-borne microbial disease.
Visceral leishmaniasis is the most virulent form of the disease, which is caused by protozoa of the genus Leishmania. The parasite is transported by several genera of flying insects and introduced into the skin by insect bites. The initial symptom is skin ulcers, followed by fever, low red blood count, and enlarged internal organs. It is a prevalent disease among poor, malnourished populations.
Over the years, Indian scientists observed that in areas where the drinking water is contaminated with As(III), Individuals not only suffer from arsenic toxicity, but they do not respond well to the leishmaniasis treatment. The Sb(V) in stibogluconate is reduced to Sb(III) by the Leishmania parasite before the antimony disrupts the bug’s defenses; As(III) works the same way. Researchers now believe that Leishmania mutations may have caused the microbe to become resistant to As(III) and Sb(III).
In 2005, the Indian government stopped recommending the use of antimony-based drugs, but sodium stibogluconate and others continue to be used because alternatives are significantly more expensive. As a result, cure rates plunged by ≈60% between 2006 and 2010.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

