What molecule am I?

Elements in group 18 (formerly group 0) of the periodic table are “inert” no more. Until recently, helium, the “noblest” of the noble gases, was the last one not to have been made into a compound with other elements.
In early 2017, Artem R. Oganov, Xiang-Feng Dong, Hui-Tian Wang, and colleagues at research institutes in China, the United States, Russia, Italy, and Germany generated a binary compound of helium and sodium under forcing conditions. They first performed a computational study that showed that the compound Na2He should be stable at pressures greater than ≈115 GPa. And voilà, lab experiments conducted in a diamond anvil cell produced the thermodynamically stable substance.
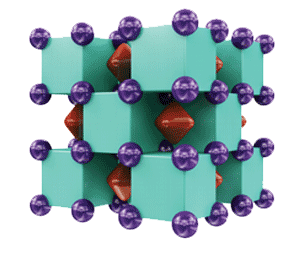
In the 3-D crystal structure shown, the purple spheres are sodium atoms, the green cubes represent helium, and the red regions denote free electrons. Because the electrons act as the compound’s anions, the structure is classified as an electride.
Although no one would expect a compound like Na2He to occur under normal atmospheric conditions, Eva Zurek at the University of Buffalo, SUNY, points out that its formation pressure is only 40% of that in the Earth’s center. So if you dig deep enough, who knows what strange substances you might find?
MOTW Update
Ketamine was the Molecule of the Week for May 25, 2015. Recently, the journal ACS Chemical Neuroscience published an extensive review of ketamine’s importance in neurochemistry.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

