What molecule am I?
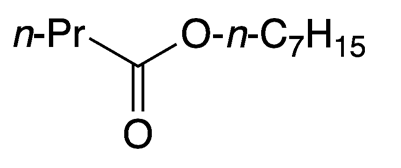
Heptyl butyrate is a colorless liquid with a chamomile-like odor that is found abundantly in fresh apples and plums. In late summer, as these fruits begin to fall from trees, swarms of yellowjackets (Vespula spp.) can appear.
In the late 1960s, Entomologist Harry G. Davis, chemist Martin Beroza, and colleagues at the US Department of Agriculture were researching attractants for the little housefly (Fannia canicularis) when they discovered the effect of heptyl butyrate on yellowjackets. But the details of this attraction were not investigated until 2016, when Peter Landolt at the USDA (Wapato, WA) and Q.H. Zhang at Sterling International (Spokane, WA) studied various potential attractants and reported that heptyl butyrate was superior to other compounds for attracting Vespula and other wasp genera.
In 2009, a fact sheet issued by the US Environmental Protection Agency stated that heptyl butyrate is a safe, food-grade compound. Earlier that year, EPA had issued a pesticide registration for the compound to be used as an attractant in yellowjacket traps and other devices.
Heptyl butyrate also occurs in babaco (an Ecuadorean hybrid papaya-like fruit in the genus Vasconcellea). So if you like the smell of fresh fruit, you and yellowjackets are in good company.
Heptyl butyrate hazard information
| Hazard class* | Hazard statement | |
|---|---|---|
| Flammable liquids, category 4 | H227—Combustible liquid | |
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
*Globally Harmonized System of Classification and Labeling of Chemicals.
Explanation of pictograms.
Heptyl butyrate fast facts
| CAS Reg. No. | 5870-93-9 |
| SciFinder nomenclature | Butanoic acid, heptyl ester |
| Empirical formula | C11H22O2 |
| Molar mass | 186.29 g/mol |
| Appearance | Colorless liquid |
| Boiling point | 226 ºC |
| Water solubility | 33 mg/L (est.) |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

