What molecule am I?
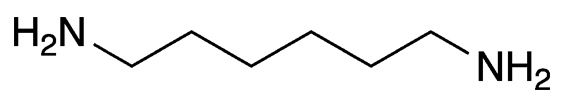
Hexamethylenediamine (formally hexane-1,6-diamine) is a colorless, low-melting solid with an important industrial use. It and adipic acid (Molecule of the Week for February 9, 2015) are the starting materials for manufacturing nylon 6,6, a polyamide used widely in textiles and plastics.
The earliest synthesis of hexamethylenediamine is attributed to Theodor Curtius and Hans Clemm, Heidelberg University (Germany) chemists, who in 1900 made it by hydrogenating adiponitrile. In 1929, biochemists Karl H. Slotta and R. Tschesche at the University of Breslau (Germany) improved this process by generating hydrogen in situ from sodium metal and ethanol.
Nylon 6,6 market value in 2018 is estimated to be more than US$3 billion; this value is expected to double by 2026. For more on the status of the nylon 6,6 industry, see the October 8, 2018, issue of Chemical & Engineering News.
Hexamethylenediamine hazard information
| GHS classification*: acute toxicity, oral, category 4 | |
| H302—Harmful if swallowed | |
| GHS classification: Acute toxicity, dermal, category 4 | |
| H312—Harmful in contact with skin | |
| GHS classification: skin corrosion, category 1B | |
| H314—Causes severe skin burns and eye damage | |
| GHS classification: serious eye damage, category 1 | |
| H318—Causes serious eye damage | |
| GHS classification: specific target organ toxicity, single exposure; respiratory tract irritation, category 3 | |
| H335—May cause respiratory irritation | |
| GHS classification: hazardous to the aquatic environment, acute hazard, category 3 | |
| H402—Harmful to aquatic environment, category 3 | |
*Globally Harmonized System of Classification and Labeling of Chemicals. Explanation of pictograms.
Hexamethylenediamine fast facts
| CAS Reg. No. | 124-09-4 |
| Empirical formula | C6H16N2 |
| Molar mass | 116.21 g/mol |
| Appearance | Colorless crystals or clear liquid |
| Melting point | 42 ºC |
| Boiling point | 205 ºC |
| Water solubility | 490 g/L |
MOTW update
L-Tartaric acid was the Molecule of the Week for November 10, 2008. It is a wine industry byproduct that is used as a food additive and industrial chemical. Tartaric acid is also important in the history of chemistry because Louis Pasteur, who most people think of mainly as a biologist, used it to demonstrate molecular chirality. Pasteur’s notebooks that described his work, however, turned up missing after his death. For and account of how the “lost” notebooks were found, see this week’s issue of Chemical & Engineering News.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

