What molecule am I?
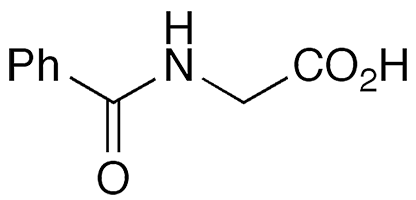
Hippuric acid, or N-benzoylglycine, is an amino acid derivative found in the urine of herbivorous animals. The name is derived from Greek hippos (horse) and ouron (urine). In 1829, the pioneering German chemist Justus von Liebig so named it because his research on the compound concentrated on horse urine.
Hippuric acid also occurs in avocadoes (Persea americana) and common beans (Phaseolus vulgaris). As its chemical name suggests, the compound is formed by the reaction of benzoic acid and glycine in the urine.
Later in the 19th century, von Liebig’s last student, Oscar Loew, also studied hippuric acid. In 1879, Loew wrote about the source of the acid in herbivores’ urine. According to an account of the article in the first volume of the Journal of the American Chemical Society (1879), quinic acid1 in hay turns into hippuric acid during digestion. This finding had previously been observed in 1863 by Eduard Lautemann, a student of another important German chemist, Hermann Kolbe.
Synthetic hippuric acid is produced similarly to the biochemical reaction: Glycine is acylated with benzoyl chloride in the presence of base, followed by acidification to form the acid. Hydrolysis in strong base restores it to glycine and benzoic acid.
Extensive information about the biochemistry of hippuric acid and its implications for human health can be found in the ScienceDirect entry on the compound.
1. CAS Reg. No. 77-95-2.
Hippuric acid hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 4 | H302—Harmful if swallowed | |
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Serious eye damage/eye irritation, category 1 | H318—Causes serious eye damage | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
*Compilation of multiple safety data sheets. Some sources report “not a hazardous substance or mixture”.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
Molecules from the journals
(–)-Illisimonin A1 is a caged sesquiterpenoid found in the fruit of Illicium simonsii, a flowering plant that grows in southern China. It was first isolated in 2017 by Jing Qu, Shi-Shan Yu, and colleagues at the Chinese Academy of Medical Sciences/Peking Union Medical College (Beijing), who also reported its structure and absolute configuration and described its neuroprotective effects against oxygen-glucose deprivation–induced SH-SY5Y cell injury.
Two years later, Alexander S. Burns and Scott D. Rychnovsky* at the University of California, Irvine, reported a revised structure of (–)-illisimonin A and the first synthesis of the racemic compound. They also confirmed its neuroprotective activity. Then, this past March, Markus Kalesse and co-workers at Leibniz University Hannover (Germany) described the total synthesis of the natural compound, which included a Ti(III)-mediated cyclization and a semipinacol rearrangement as the key steps.
A catenane is a molecule in which two individual ring compounds are intertwined. In 1960, Edel Wasserman at the Bell Telephone Laboratories (Murray Hill, NJ, now Nokia Bell Labs) reported the first preparation of a catenane, which consisted of a cyclotetratriacontane ring that contained five deuterium atoms2 interlocked with the acyloin 2-hydroxycyclotetratriacontanone3. The assemblage soon became known as Wasserman's catenane4.
Wasserman’s method is known as the “statistical approach” to catenanes, in which the ring closure of one molecule (in this case, the acyloin) within the other (the hydrocarbon) is essentially random, resulting in a small yield of the catenane. The analytical methods available to Wasserman could not affirm the existence of a catenane, so Wasserman was obliged to use indirect chemical methods to determine the structure. Consequently, Wasserman’s claim of synthesizing a catenane was under suspicion for more than 60 years.
In April, David A. Leigh and collaborators at the University of Manchester (UK) and East China Normal University (Shanghai) reported the results of their effort to verify the structure of Wasserman's catenane. They used modern tools such as tandem mass spectrometry, deuterium NMR spectroscopy, and fluorescent tag labeling to prove that the two 34-member rings are indeed linked, thus confirming Wasserman’s claim.
For additional information about Wasserman's catenane, see recent articles by Derek Lowe and Andy Extance.
1. CAS Reg. No. 2162197-76-2.
2. CAS Reg. No. 119212-00-9.
3. CAS Reg. No. 119211-99-3.
4. CAS Reg. No. 119212-01-0.
Molecules from the Journals
MOTW briefly describes noteworthy molecules that appeared in recent ACS journal articles. See this week's
edition below.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Hippuric acid fast facts
| CAS Reg. No. | 495-69-2 |
| SciFinder nomenclature | Glycine, N-benzoyl- |
| Empirical formula | C9H9NO3 |
| Molar mass | 179.17 g/mol |
| Appearance | White crystals or powder |
| Melting point | 191 °C |
| Water solubility | ≈4 g/L |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

