What molecule am I?
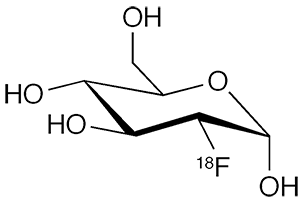
2-Deoxy-2-fluoro-D-glucose1, a fluorinated derivative of glucose, was first synthesized in 1969 by Josef Pacák*, Zdeněk Točik, and Miloslav Černý at Charles University (Prague) in a four-step sequence beginning with 1,6-anhydro-4-O-benzyl-2-O-(p-tolylsulfonyl)-β-D-glucopyranose2, which they had prepared the previous year. In 1993, Stephen G. Withers and co-workers at the University of British Columbia (Vancouver) improved upon this synthesis by treating a peracetate derivative of 2-deoxy-D-glucose with acetyl hypofluorite3.
But the real story here is the radioactive form of 2-deoxy-2-fluoro-D-glucose, 2-deoxy-2-(fluoro-18F)-D-glucose. The 18fluorine isotope is a positron emitter that has become useful in medical imaging via positron emission tomography (PET) as a marker for glucose in the body.
2-Deoxy-2-(fluoro-18F)-D-glucose was synthesized in 1978 by Tatsuo Ido, Alfred P. Wolf, and co-workers at the Brookhaven National Laboratory (Upton, NY). They used a method different from that of Pacák et al: 3,4,6-Tri-O-acetyl-D-glucal4 was treated with 18F-labeled molecular fluorine, followed by hydrolysis to give the desired labeled compound and the equivalent mannose derivative. For good measure, the authors also prepared a 14C-labeled version of 2-deoxy-2-fluoro-D-glucose.
The application of 2-deoxy-2-(fluoro-18F)-D-glucose for glucose tracing with PET is especially useful in tumor imaging. The technique, in combination with computed tomography (CT) scanning, is also used for evaluating glucose metabolism in the heart and brain. These, along with other applications, are described in the more than 45,000 citations for the molecule in Chemical Abstract Service’s SciFinder.
1. CAS Reg. No. 29702-43-0.
2. CAS Reg. No. 20183-65-7.
3. CAS Reg. No. 78948-09-1.
4. CAS Reg. No. 2873-29-2.
2-Deoxy-2-fluoro-D-glucose hazard information*
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
*Hazard information unavailable for 2-deoxy-2-(fluoro-18F)-D-glucose does not account for hazards associated with radioactivity.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
Molecule from the Journals: Hazard Alert
N-Bromosuccinimide1 (NBS) is a valuable reagent in organic chemistry. It is primarily used in electrophilic and radical bromination reactions. Its use was established in an extensive 1942 article titled “halogenation of unsaturated substances in the allyl position” by Karl Ziegler and co-workers at the University of Halle–Saale (Germany; now Martin Luther University Halle–Wittenberg). Ziegler and Italian chemical engineer Giulio Natta won the 1963 Nobel Prize in Chemistry for their pioneering work in polymers.
Since its introduction, NBS has been used widely and safely. But last month, Elsa M. Gonçalves and colleagues at Hovione FarmaCiencia (Lisbon) reported that when a 22% solution of NBS in N,N-dimethylformamide2 (DMF) was heated to 80 °C, it decomposed suddenly and rapidly to give off a gas consisting of decomposition products of both compounds. After an extensive investigation of the decomposition reaction, the authors concluded that the maximum safe working temperature for solutions of NBS in DMF under industrial conditions is 32 °C.
1. CAS Reg. No. 128-08-5.
2. CAS Reg. No. 68-12-2.
Molecule from the Journals: Hazard Alert
MOTW briefly describes noteworthy molecules that appeared in recent ACS journal articles. See this week's special edition below.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
2-Deoxy-2-(fluoro-18F)-D-glucose fast facts
| CAS Reg. No. | 63503-12-8 |
| SciFinder nomenclature | D-Glucose, 2-deoxy-2-(fluoro-18F)- |
| Empirical formula | C6H1118FO5 |
| Molar mass | 181.15 g/mol |
| Appearance | White crystals or powder |
| Melting point | 174–176 °Ca |
| Water solubility | 10 g/La |
a. Data for 2-deoxy-2-fluoro-D-glucose.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

