What molecule am I?
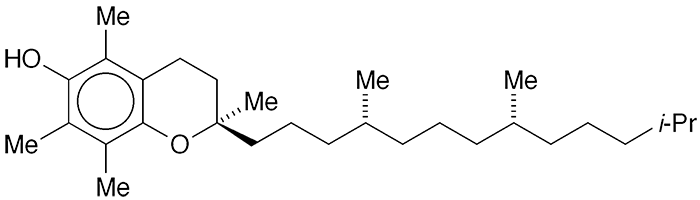
(+)-α-Tocopherol is one of eight components of vitamin E and, while not the most abundant, it is the most biologically active. It occurs naturally in many foods, especially in plant oils such as wheat germ, canola, sunflower, and safflower.
In 1936, Herbert M. Evans*, Oliver H. Emerson, and Gladys A. Emerson at the University of California, Berkeley, reported the first isolation of α-tocopherol, from wheat germ. Vitamin E was already known, and these authors established that α-tocopherol is its active component. Between 1938 and 1943, the same team published seven papers on the compound’s role in nutrition.
Also in 1938, Erhard Fernholz at Merck (Rahway, NJ) described the structure of α-tocopherol, which he deduced from its degradation products. The same year, three other research groups reported syntheses of the molecule: Paul Karrer et al. at the University of Zurich, Franz Bergel et al. at the University of Manchester (UK), and Lee Irvin Smith et al. at the University of Minnesota (Minneapolis). Five years later, Smith and Joseph A. Sprung followed up with an improved synthesis.
The main biological function of vitamin E/α-tocopherol is as a fat-soluble antioxidant. The US National Academy of Medicine’s adult daily dietary recommendation for vitamin E is 15 mg; individuals with deficiencies are often prescribed the vitamin as a supplement. Vitamin E is also added to commercial fats and oils as an antioxidant.
During this National Chemistry Week, “The Healing Power of Chemistry”, keep in mind how α-tocopherol keeps your body safe.
α-Tocopherol hazard information
| Hazard class* | GHS code and hazard statement |
|---|---|
| Not a hazardous substance or mixture** |
*Globally Harmonized System (GHS) of Classification and Labeling of Chemicals.
**Most safety data sheets give this description; but one lists four hazard classes.
Molecule of the Future
The dictyodendrins are highly aromatic alkaloids that occur in the Japanese marine sponge Dictyodendrilla verongiformis. The first five dictyodendrins (A–E) were isolated in 2002 by Nobuhiro Fusetani and collaborators at the Universities of Tokyo and Amsterdam. These authors also determined the molecules’ structures and showed that they completely inhibit telomerase1 at a concentration of 50 μg/mL. In 2012, Robert J. Capon and co-workers at the University of Queensland (St. Lucia, Australia) isolated five additional dictyodendrins (F–J) from a southern Australian sponge in the Ianthella genus.
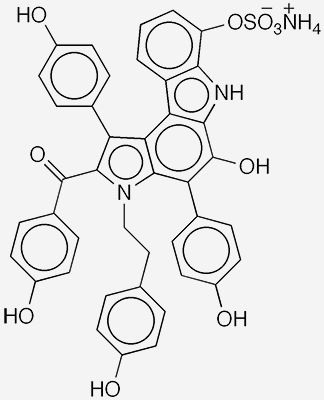
This past April, Yuya Okui, Atsunori Mori, and Kentaro Okano* at Kobe University (Japan) reported what they described as a “formal synthesis” of dictyodendrin B2 (see image). The key step in the synthesis was the formation of the internal benzene ring in the tetracyclic pyrrolo[2,3-c]carbazole section of the molecule. This synthesis could be an important accomplishment in the development of future cancer treatments.
1. CAS Reg. No. 120178-12-3. Telomerase is a ribonucleoprotein enzyme that adds repeats of the DNA sequence TTAGGG, called telomeres, onto the 3‘-ends of chromosomes. Telomerase activity is found in ≈90% of human tumors, but not in normal cells.
2. CAS Reg. No. 510709-69-0.
Molecule of the Future
Once a month we bring you a newly discovered or developed molecule that has important implications for the future of chemistry or society in general. Look for it the third week of each month. Learn more about this month's Molecule of the Future below.
We're looking for more molecules of the future!
Do you have a suggestion for the next molecule of the future? Send your idea to MOTW.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
α-Tocopherol fast facts
| CAS Reg. No. | 59-02-9 |
| SciFinder nomenclature | 2H-1-Benzopyran-6-ol, 3,4-dihydro-2,5,7,8-tetramethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-, (2R)- |
| Empirical formula | C29H50O2 |
| Molar mass | 430.71 g/mol |
| Appearance | Pale yellow viscous liquid |
| Melting point | 210 °C (0.1 Torr) |
| Water solubility | 21 mg/L (33 °C) |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

