What molecule am I?
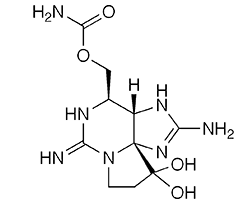
Saxitoxin is a pyrrolopurine alkaloid and potent neurotoxin found in a variety of shellfish, including mussels, clams, and scallops. It is produced by marine dinoflagellates (e.g., Gonyaulax catenella and Alexandrium tamarense), freshwater cyanobacteria (e.g., Dolichospermum cicinale), and other microorganisms that the shellfish ingest. The term saxitoxin is also used for a series of structurally related microbe-derived neurotoxins.
Saxitoxin takes its name from the Alaskan butter clam (Saxidomus gigantea) from which it was first identified in 1937 by Hermann Sommer1 and co-workers at the University of California, San Francisco. In 1966, Edward J. Schantz1 and colleagues at the US Army Biological Laboratories (Fort Detrick, MD) purified saxitoxin and determined its properties. By 1975, Schantz was at the University of Wisconsin (Madison); he and collaborators there and at Iowa State University (Ames) elucidated the structure of saxitoxin.
In 2008, Mark A. Simmons at Northeast Ohio Medical University (Rootstown) wrote:
Saxitoxin (STX), a highly selective and potent blocker of voltage-dependent sodium channels in motor nerves, causes skeletal muscle paralysis. It is found in filter-feeding mollusks [that] consume planktonic algae. . . . Ingestion of these mollusks by humans results in paralytic shellfish poisoning, which may result in death in a matter of hours if sufficient toxin is absorbed. There is no antidote for STX poisoning.
Also in 2008, Brian A. Neilan and co-workers at the University of New South Wales (Sydney) proposed a 10-step biosynthetic pathway to saxitoxin in the freshwater cyanobacterium Cylindrospermopsis raciborskii. For much more information about saxitoxin, see the ScienceDirect information page.
1. Sommer and Schantz were pioneers in characterizing shellfish toxins.
Saxitoxin hazard information
| Hazard class* | GHS code and hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 1, 2 | H300—Fatal if swallowed | |
| Acute toxicity, dermal, category 1, 2 | H310—Fatal in contact with skin | |
| Acute toxicity, inhalation, category 1, 2 | H330—Fatal if inhaled | |
*Globally Harmonized System (GHS) of Classification and Labeling of Chemicals. Explanation of pictograms.
Molecules in the News
Pseudouridine1 is a ribonucleoside and an isomer of the more frequently encountered uridine2. The two differ by the location of the bond that connects their uracil and ribofuranose rings. Both are common components of RNA, but not DNA.
Waldo E. Cohn at Oak Ridge National Laboratory (TN) pioneered research into pseudouridine. In 1960, he described the isolation of its phosphates from hydrolyzed RNA, along with the determination of its structure and chemical properties.
In recent years, pseudouridine proved to be a key molecule in the development of mRNA-based COVID-19 vaccines. In 2005, Katalin Karikó at the University of Szeged (Hungary), Drew Weissman at the University of Pennsylvania (Philadelphia), and colleagues, in their attempts to use RNA to produce vaccines that could quickly be adapted to specific diseases, found that converting uridine to pseudouridine reduced the inflammatory response of mRNA while maintaining the immune response. Karikó, Weissman, et al. later showed that the switch to pseudouridine produced more stable RNA constructs and better protein yields.
Karikó and Weissman’s findings paid off big time when the necessity arose to produce COVID-19 vaccines in a hurry. For their groundbreaking work, they were awarded the 2023 Nobel Prize in Physiology or Medicine.
Erythrosine3, also known as FD&C Red No. 3, is a US Food and Drug Administration–approved food coloring additive and biological stain. It has been known since the late 19th century; in an 1899 article, Joseph W. Ellms at the Cincinnati Water Works laboratory reported its use as an indicator for determining the alkalinity of water.
In the 1970s, erythrosine was identified as a potential carcinogen. Since then, it has been outlawed as a food additive in many developed nations. It is still legal in the United States, but some states have considered banning it. This month, California prohibited it and three other food ingredients effective January 1, 2027.
1. CAS Reg. No. 1445-07-4.
2. CAS Reg. No. 58-96-8.
3. CAS Reg. No. 16423-68-0.
Molecules in the News
MOTW highlights molecules that appear in major news outlets. See this week's edition below.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Saxitoxin fast facts
| CAS Reg. No. | 35523-89-8 |
| SciFinder nomenclature | 1H,10H-Pyrrolo[1,2-c]purine-10,10-diol, 2,6-diamino-4-{[(aminocarbonyl) oxy]methyl}-3a,4,8,9-tetrahydro-, (3aS,4R,10aS)- |
| Empirical formula | C10H17N4O4 |
| Molar mass | 299.29 g/mol |
| Appearance | White hygroscopic powder |
| Melting point | Not reported |
| Water solubility | Very soluble |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

