What molecule am I?
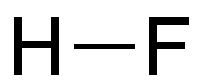
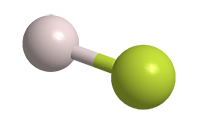
Hydrogen fluoride (HF) is an extremely toxic, corrosive gas and liquid. As the lightest of the hydrogen halides, it has a surprisingly high boiling point, higher even than that of hydrogen iodide. The reason is the strong electronegativity of the fluorine atom, which causes hydrogen and fluorine atoms to form hydrogen bonds in the liquid phase.1 The other hydrogen halides are connected by much weaker van der Waals forces.
Nomenclature confusion?
The molecule HF is commonly called hydrogen fluoride; but its Chemical Abstracts Service name, as found in SciFinder, is hydrofluoric acid. This usage also applies to the other hydrogen halides and has the potential for confusion with the terminology for aqueous solutions of the same compounds.
In aqueous solution, HF is a weak acid, with a pKa of 3.17, again in contrast with the other hydrogen halides, which are all completely ionized in solution. But there is nothing weak about HF’s other properties. As a gas, as a liquid, or in solution, it is extremely aggressive toward a wide range of substances, including most metals, glass and other forms of silica, and animal and plant tissue.
French chemist Edmond Frémy discovered HF gas in the mid-19th century, although HF solutions had been known and used at least 100 years earlier. Its main source today is the mineral fluorite (calcium fluoride), which is treated with sulfuric acid to form HF gas. Significant amounts of HF also are obtained as a byproduct of phosphate fertilizer manufacture from the mineral apatite, which contains fluoride ion as well as phosphate.
The synthesis of almost all commercial fluorine-containing chemicals begins with HF. These include inorganics, such as molecular fluorine (F2), cryolite (Na3AlF6) for aluminum production, and a wide range of metal fluorides; and organics as diverse as polymers (e.g., polytetrafluoroethylene, or Teflon), chlorofluorocarbon refrigerants, and perfluoroalkyl substances (PFAS), which currently are in the news as notorious environmental contaminants. HF itself is used as a catalyst in petroleum refining.
A more modern use of HF is in chemical lasers. George C. Pimentel at the University of California, Berkeley, a pioneer in the field, developed dozens of infrared lasers based on chemical reactions, including some that use the reaction of fluorine and hydrogen to form HF. HF lasers emit IR light in the 2.7–2.9 µm wavelength range; if the hydrogen is replaced by deuterium, the wavelength increases to ≈3.8 µm.
1. The hydrogen bonds are so strong that HF can form homopolymers, designated as (HF)n.
Hydrogen fluoride hazard information
| Hazard class* | GHS code and hazard statement | |
|---|---|---|
| Gases under pressure, liquefied gas | H280—Contains gas under pressure; may explode if heated | |
| Acute toxicity, oral, category 2 | H300—Fatal if swallowed | |
| Acute toxicity, dermal, category 1 | H310—Fatal in contact with skin | |
| Skin corrosion/irritation, category 1A | H314—Causes severe skin burns and eye damage | |
| Serious eye damage/eye irritation, category 1 | H318—Causes serious eye damage | |
| Acute toxicity, inhalation, category 2 | H330—Fatal if inhaled | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
| Simple asphyxiant | H380**—May displace oxygen and cause rapid suffocation | |
*Globally Harmonized System (GHS) of Classification and Labeling of Chemicals.
Explanation of pictograms.
**Not an official GHS code.
Hydrogen fluoride
fast facts
| CAS Reg. No. | 7664-39-3 |
| SciFinder nomenclature | Hydrofluoric acid |
| Empirical formula | HF |
| Molar mass | 20.01 g/mol |
| Appearance | Colorless gas |
| Boiling point | 19.5 °C |
| Water solubility | Miscible |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.


