What molecule am I?
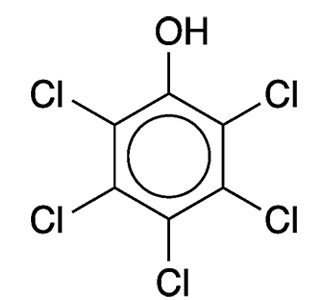
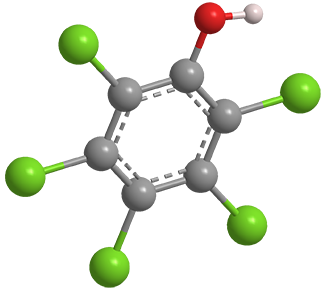
Pentachlorophenol is an old-line pest control agent that came under regulatory pressure decades ago. Beginning in the 1930s, it was used as an insecticide (including for termite control), a defoliant for crops before they were harvested, a wood preservative (notably in telephone poles), and a biocide with numerous other agricultural and industrial uses.
Pentachlorophenol is made by chlorinating phenol at high temperature. In the commercial process, chlorination is incomplete, and the product contains even more toxic impurities such as dibenzo-p-dioxin derivatives. The compound’s odor is similar to that of benzene or phenol; it is sparingly soluble in water and slow to decompose in nature.
Pentachlorophenol’s pervasive toxicity and environmental threats (see the hazard information table) doomed it to severe use restrictions by the 1980s. Today, wood preservation is the only use permitted by the US Environmental Protection Agency. Earlier this year, the EPA proposed to ban it completely. The comment period for this proposal expired in May, and EPA is currently proceeding with canceling the registration
For additional information, see EPA’s information page on pentachlorophenol.
Pentachlorophenol hazard information
| Hazard class* | GHS code and hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 3 | H301—Toxic if swallowed | |
| Acute toxicity, dermal, category 3 | H311—Toxic in contact with skin | |
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Acute toxicity, inhalation, category 2 | H330—Fatal if inhaled | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
| Carcinogenicity, category 2 | H351—Suspected of causing cancer | |
| Short-term (acute) aquatic hazard, category 1 | H400—Very toxic to aquatic life | |
| Long-term (chronic) aquatic hazard, category 2 | H411—Toxic to aquatic life with long-lasting effects | |
*Globally Harmonized System (GHS) of Classification and Labeling of Chemicals.
Explanation of pictograms.
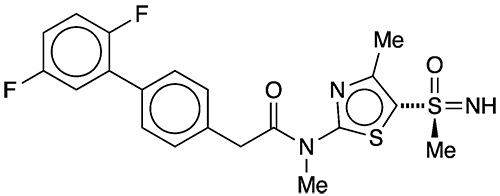
Now, Innovative Molecules (Bad-Salzuflen, Germany) reports that a new small molecule, IM-2501, combats active and latent HSV in guinea pigs without inducing adverse side effects that plagued other drugs investigated for this purpose. The company’s CEO expects that IM-250 will begin Phase 1 clinical trials in late 2022 or early 2023.
1. (S)-2-[2′,5′-difluoro-(1,1′-biphenyl)-4-yl]-N-methyl-N-[4-methyl-5-(S-methylsulfon-imidoyl)thiazol-2-yl]acetamide.
Molecule of the Future
Once a month we bring you a newly discovered or developed molecule that has important implications for the future of chemistry or society in general. Look for it the third week of each month. Learn more about this month's Molecule of the Future below.
We're looking for more molecules of the future!
Do you have a suggestion for the next molecule of the future? Send your idea to MOTW.
Pentachlorophenol
fast facts
| CAS Reg. No. | 87-86-5 |
| SciFinder nomenclature | Phenol, 2,3,4,5,6-pentachloro- |
| Empirical formula | C6HCl5O |
| Molar mass | 266.34 g/mol |
| Appearance | White crystals or powder |
| Melting point | 191 °C |
| Water solubility | 14 mg/L |
MOTW update
Nitrous oxide (N2O) was the Molecule of the Week for January 14, 2013. It is the third-most-emitted climate change contributor, after carbon dioxide and methane, yet little has been done to curtail it. Now, pressure from government agencies and environmental groups is forcing the chemical industry worldwide to install equipment to abate N2O emissions.
MOTW update:
February 21, 2022
Pentachlorophenol is an old-line insecticide and wood preservative that is a “reasonably anticipated” human carcinogen and an environmental hazard.
After decades of restricting the use of pentachlorophenol, the US Environmental Protection Agency proposed a complete ban on it in early 2021. This month, EPA followed through on the ban, which will go into effect in 2024. Wood-treatment companies are permitted to use up existing stocks until 2027.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

