What molecule am I?
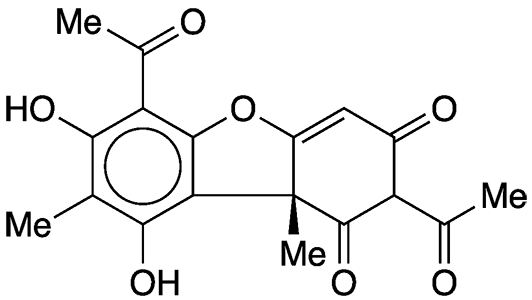
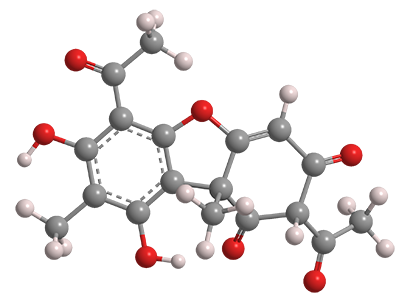
Usnic acid is a natural antibacterial compound found in lichens. It is not a carboxylic acid; instead, it gets acidity from the phenolic hydroxyl groups. Its pKa is 4.4.
In the 1840s, German and Austrian scientists isolated yellow-colored usnic acid from several lichen genera, such as Usnea, from which it derives its name. The bitter-tasting acid exists naturally as its (R)- and (S)-enantiomers as well as the racemate. (R)-Usnic acid is shown in the 3-D image.
More than 90 years after its was isolated, usnic acid’s structure was elucidated by Frank H. Curd and Alexander Robertson* at the University of Liverpool (UK). In a long series of articles, these authors reported the structure and laboratory synthesis of usnic acid and many of its derivatives.
As shown in the hazard information table, usnic acid is hazardous to health and the environment. Nevertheless, it is an ingredient in some over-the-counter dietary supplements. The Memorial Sloan Kettering Cancer Center, among other reputable organizations, warns against using it.
Even with its hazards, usnic acid is being studied for medical applications, especially in cancer research. In 2012, Helga M. Ogmundsdottir and colleagues at the University of Iceland (Reykjavik) reported that it affects mitochondrial and lysosomal function in cancer cells and has “implications for therapeutic manipulation of autophagy and pH-determined drug distribution.”
More recently, in 2018, Wensheng Pan and co-workers at Zhejiang University and People’s Hospital of Hangzhou Medical College (both in Hangzhou, China) found that usnic acid induces cycle arrest, apoptosis, and autophagy in gastric cancer cells.
Usnic acid hazard information
| Hazard class** | GHS code and hazard statement | |
|---|---|---|
| Acute toxicity, oral, category 3 | H301—Toxic if swallowed | |
| Skin corrosion/irritation, category 2 | H315—Causes skin irritation | |
| Skin sensitization, category 1 | H317—May cause an allergic skin reaction | |
| Serious eye damage/eye irritation, category 2A | H319—Causes serious eye irritation | |
| Specific target organ toxicity, single exposure, respiratory tract irritation, category 3 | H335—May cause respiratory irritation | |
| Short-term (acute) aquatic hazard, category 1 | H400—Very toxic to aquatic life | |
| Long-term (chronic) aquatic hazard, category 1 | H410—Very toxic to aquatic life with long-lasting effects | |
*Compilation of multiple safety data sheets.
**Globally Harmonized System (GHS) of Classification and Labeling of Chemicals.
Explanation of pictograms.
This molecule was suggested by a reader. We present almost all of the molecules suggested by our readers. If you have a molecule you would like us to consider, please send us a message. And thank you for your interest in Molecule of the Week! —Ed.
Usnic acid fast facts
| CAS Reg. No. | 125-46-2 |
| SciFinder nomenclature | 1,3(2H,9bH)-Dibenzofurandione, 2,6-diacetyl-7,9-dihydroxy-8,9b-dimethyl- |
| Empirical formula | C18H16O7 |
| Molar mass | 344.32 g/mol |
| Appearance | Yellow crystals or powder |
| Melting point | 204 °C |
| Water solubility | ≈1 g/L |

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

