What molecule am I?
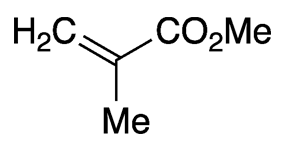
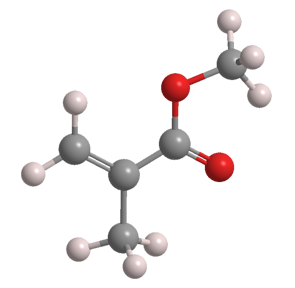
Methyl methacrylate (MMA) is an unsaturated ester that has several uses in polymer manufacturing. It is a clear liquid with a characteristic ester odor.
There are several synthetic routes to MMA. The most widely used are a three-step sequence that begins with acetone and HCN and proceeds through acetone cyanohydrin; and a two-step process that begins with the reaction of ethylene and methanol to produce methyl propionate.
By far the greatest volume of MMA is polymerized to the homopolymer poly(methyl methacrylate) (PMMA). The polymer is a clear plastic that is known by the familiar trade names Lucite and Plexiglas.
MMA is also copolymerized with styrene and butadiene to make an additive to poly(vinyl chloride) (PVC) that improves its properties. MMA is also transesterified to make specialty methacrylate monomers.
Methyl methacrylate hazard information
| GHS classification*: flammable liquids, category 2 | |
| H225—Highly flammable liquid and vapor | |
| GHS classification: skin irritation, category 2 | |
| H315—Causes skin irritation | |
| GHS classification: skin sensitization, category 1 | |
| H317—May cause an allergic skin reaction | |
| GHS classification: specific target organ toxicity, single exposure; respiratory tract irritation, category 3 | |
| H335—May cause respiratory irritation | |
| GHS classification: hazardous to the aquatic environment, acute hazard, category 3 | |
*Globally Harmonized System of Classification and Labeling of Chemicals. Explanation of pictograms.
Methyl methacrylate
fast facts
| CAS Reg. No. | 80-62-6 |
| Molar mass | 100.12 g/mol |
| Empirical formula | C5H8O2 |
| Appearance | Colorless liquid |
| Boiling point | 101 ºC |
| Water solubility | 15 g/L |
MOTW update:
April 3, 2023
Methyl methacrylate1 (MMA) is an unsaturated ester that is used to make polymers that range from water-based latexes to clear plastics.
On March 24, 2023, MMA and two other latex monomers, butyl acrylate2 and ethyl acrylate3, were released into the Delaware River during a discharge from a polymer manufacturing plant in Bristol, PA. All three monomers are respiratory and dermal hazards. On March 26, authorities stated that the tap water in Philadelphia and surrounding southeastern Pennsylvania communities was safe to drink; but they advised residents to use bottled drinking water. The result was panic buying that left store shelves empty. As of this writing, Philadelphia tap water has not been shown to be contaminated.
1. CAS Reg. No. 80-62-6.
2. CAS Reg. No. 141-32-2.
3. CAS Reg. No. 140-88-5.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

