What molecule am I?
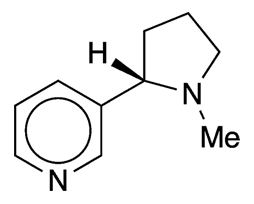
L-(–)-Nicotine is a dinitrogen alkaloid that is present in concentrations as high as 3% in the dried leaves of the tobacco plant (Nicotiana tabacum). It exists in even higher concentrations (up to 14%) in the lesser known “Aztec tobacco” (N. rustica).
Nicotine is an unusual alkaloid in that it has two nitrogen-containing heterocycles, pyridine and pyrrolidine. It is, of course, the tobacco component that makes smoking highly addictive, leading to the consequence that long-term smoking causes cancer. The now-famous 1964 Report of the Advisory Committee to the Surgeon General of the United States brought widespread attention to the dangers of smoking.
In the modern era, much nicotine is delivered via e-cigarettes. Smokers have found that the nicotine in e-cigarettes is not as harsh to inhale as the one contained in tobacco.
A recent report by David H. Peyton and co-workers at Portland State University (Oregon) suggests why this is so: Nicotine in e-cigarettes is primarily in its protonated form rather than the free base that exists in tobacco. The authors infer that protonated nicotine is easier to inhale. Thus, if anything, e-cigarettes are likely to increase nicotine addiction
If you think nicotine is bad when you inhale it, take a look at its acute effects shown in the hazard information box.
Nicotine hazard information
| GHS classification*: acute toxicity, oral, category 2 | |
| H300—Fatal if swallowed | |
| GHS classification: acute toxicity, dermal, category 1 | |
| H310—Fatal in contact with skin | |
| GHS classification: hazardous to the aquatic environment, acute hazard, category 1 | |
| H400—Very toxic to aquatic life | |
| GHS classification: hazardous to the aquatic environment, long-term hazard, category 1 | |
| H410— Very toxic to aquatic life with long-lasting effects | |
*Globally Harmonized System of Classification and Labeling of Chemicals. Explanation of pictograms.
Molecule in the News: April 28, 2025
Nicotine1, the Molecule of the Week for August 20, 2018, is the well-known addictive component of tobacco. In February, Patricia J. Zettler, Theodore L. Wagener, and Micah L. Berman* at Ohio State University (Columbus) asked, “What’s Next for Nicotine? The Coming Legal and Political Battles over an FDA Proposal”. In the final days of the Biden administration, the US Food and Drug Administration proposed a substantial reduction in the nicotine content of cigarettes and other tobacco products to reduce their addictiveness. But, because of uncertainty about Trump administration health policy and anticipated strong pushback by the tobacco industry, the proposal is in jeopardy. The authors nevertheless advocated for the reduction to be adopted and urge medical and public health researchers to “submit . . . comments on the proposed rule to supplement the already robust evidence supporting it, countering anticipated arguments against the rule, and identifying ways to prevent industry evasion of a nicotine standard, should one be implemented.”
1. CAS Reg. No. 54-11-5.
Molecules in the News
MOTW highlights molecules that appear in major news outlets. See this week's edition.
Nicotine fast facts
| CAS Reg. No. | 54-11-5 |
| Molar mass | 162.23 g/mol |
| Empirical formula | C10H14N2 |
| Appearance | Colorless to yellow-brown oily liquid |
| Boiling point | 247 ºC |
| Water solubility | ≈16 g/L |
MOTW update
Chlorpyrifos was the Molecule of the Week for November 8, 2010. It was once a widely used insecticide, but since 2001 its use has been increasingly restricted. In the latest development, the US 9th Circuit Court of Appeals chastised the US Environmental Protection Agency for its failure to act on findings of chlorpyrifos’ harm to children’s brains and ordered the Trump administration to ban the pesticide within 60 days.

Learn more about this molecule from CAS, the most authoritative and comprehensive source for chemical information.
Molecule of the Week needs your suggestions!
If your favorite molecule is not in our archive, please send us a message. The molecule can be notable for its current or historical importance or for any quirky reason. Thank you!
Stay Ahead of the Chemistry Curve
Learn how ACS can help you stay ahead in the world of chemistry.

